Ex Vivo Bioactivity and HIV-1 Latency Reversal by Ingenol Dibenzoate and Panobinostat in Resting CD4(+) T Cells from Aviremic Patients
- PMID: 26169416
- PMCID: PMC4576101
- DOI: 10.1128/AAC.01077-15
Ex Vivo Bioactivity and HIV-1 Latency Reversal by Ingenol Dibenzoate and Panobinostat in Resting CD4(+) T Cells from Aviremic Patients
Abstract
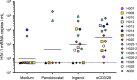
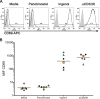
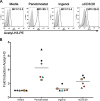
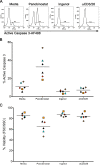
The human immunodeficiency virus type 1 (HIV-1) latent reservoir in resting CD4(+) T cells represents a major barrier to viral eradication. Small compounds capable of latency reversal have not demonstrated uniform responses across in vitro HIV-1 latency cell models. Characterizing compounds that demonstrate latency-reversing activity in resting CD4(+) T cells from aviremic patients ex vivo will help inform pilot clinical trials aimed at HIV-1 eradication. We have optimized a rapid ex vivo assay using resting CD4(+) T cells from aviremic HIV-1(+) patients to evaluate both the bioactivity and latency-reversing potential of candidate latency-reversing agents (LRAs). Using this assay, we characterize the properties of two candidate compounds from promising LRA classes, ingenol 3,20-dibenzoate (a protein kinase C agonist) and panobinostat (a histone deacetylase inhibitor), in cells from HIV-1(+) antiretroviral therapy (ART)-treated aviremic participants, including the effects on cellular activation and cytotoxicity. Ingenol induced viral release at levels similar to those of the positive control (CD3/28 receptor stimulation) in cells from a majority of participants and represents an exciting LRA candidate, as it combines a robust viral reactivation potential with a low toxicity profile. At concentrations that blocked histone deacetylation, panobinostat displayed a wide range of potency among participant samples and consistently induced significant levels of apoptosis. The protein kinase C agonist ingenol 3,20-dibenzoate demonstrated significant promise in a rapid ex vivo assay using resting CD4(+) T cells from treated HIV-1-positive patients to measure latent HIV-1 reactivation.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
In vivo analysis of the effect of panobinostat on cell-associated HIV RNA and DNA levels and latent HIV infection.Retrovirology. 2016 May 21;13(1):36. doi: 10.1186/s12977-016-0268-7. Retrovirology. 2016. PMID: 27206407 Free PMC article.
-
Histone deacetylase inhibition reduces deleterious cytokine release induced by ingenol stimulation.Biochem Pharmacol. 2022 Jan;195:114844. doi: 10.1016/j.bcp.2021.114844. Epub 2021 Nov 18. Biochem Pharmacol. 2022. PMID: 34801521 Free PMC article.
-
Innate Immune Activity Correlates with CD4 T Cell-Associated HIV-1 DNA Decline during Latency-Reversing Treatment with Panobinostat.J Virol. 2015 Oct;89(20):10176-89. doi: 10.1128/JVI.01484-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223643 Free PMC article. Clinical Trial.
-
Getting the "Kill" into "Shock and Kill": Strategies to Eliminate Latent HIV.Cell Host Microbe. 2018 Jan 10;23(1):14-26. doi: 10.1016/j.chom.2017.12.004. Cell Host Microbe. 2018. PMID: 29324227 Free PMC article. Review.
-
The development of immune-modulating compounds to disrupt HIV latency.Cytokine Growth Factor Rev. 2012 Aug-Oct;23(4-5):159-72. doi: 10.1016/j.cytogfr.2012.05.003. Epub 2012 Jul 4. Cytokine Growth Factor Rev. 2012. PMID: 22766356 Review.
Cited by
-
Addressing an HIV cure in LMIC.Retrovirology. 2021 Aug 3;18(1):21. doi: 10.1186/s12977-021-00565-1. Retrovirology. 2021. PMID: 34344423 Free PMC article. Review.
-
The ingenol-based protein kinase C agonist GSK445A is a potent inducer of HIV and SIV RNA transcription.PLoS Pathog. 2022 Jan 18;18(1):e1010245. doi: 10.1371/journal.ppat.1010245. eCollection 2022 Jan. PLoS Pathog. 2022. PMID: 35041707 Free PMC article.
-
Ongoing Clinical Trials of Human Immunodeficiency Virus Latency-Reversing and Immunomodulatory Agents.Open Forum Infect Dis. 2016 Oct 7;3(4):ofw189. doi: 10.1093/ofid/ofw189. eCollection 2016 Oct. Open Forum Infect Dis. 2016. PMID: 27757411 Free PMC article. Review.
-
Challenges and strategies for the eradication of the HIV reservoir.Curr Opin Immunol. 2016 Oct;42:65-70. doi: 10.1016/j.coi.2016.05.015. Epub 2016 Jun 9. Curr Opin Immunol. 2016. PMID: 27288651 Free PMC article. Review.
-
HIV-1 Latency and Latency Reversal: Does Subtype Matter?Viruses. 2019 Nov 28;11(12):1104. doi: 10.3390/v11121104. Viruses. 2019. PMID: 31795223 Free PMC article. Review.
References
-
- HIV-Causal Collaboration, Ray M, Logan R, Sterne JA, Hernandez-Diaz S, Robins JM, Sabin C, Bansi L, van Sighem A, de Wolf F, Costagliola D, Lanoy E, Bucher HC, von Wyl V, Esteve A, Casbona J, del Amo J, Moreno S, Justice A, Goulet J, Lodi S, Phillips A, Seng R, Meyer L, Perez-Hoyos S, Garcia de Olalla P, Hernan MA. 2010. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS 24:123–137. doi:10.1097/QAD.0b013e3283324283. - DOI - PMC - PubMed
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. doi:10.1126/science.278.5341.1295. - DOI - PubMed
-
- Chun TW, Justement JS, Murray D, Hallahan CW, Maenza J, Collier AC, Sheth PM, Kaul R, Ostrowski M, Moir S, Kovacs C, Fauci AS. 2010. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS 24:2803–2808. doi:10.1097/QAD.0b013e328340a239. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

