Biosynthetic Machinery Involved in Aberrant Glycosylation: Promising Targets for Developing of Drugs Against Cancer
- PMID: 26161361
- PMCID: PMC4479729
- DOI: 10.3389/fonc.2015.00138
Biosynthetic Machinery Involved in Aberrant Glycosylation: Promising Targets for Developing of Drugs Against Cancer
Abstract
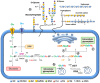
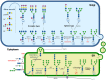
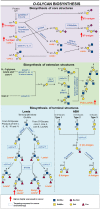
Cancer cells depend on altered metabolism and nutrient uptake to generate and keep the malignant phenotype. The hexosamine biosynthetic pathway is a branch of glucose metabolism that produces UDP-GlcNAc and its derivatives, UDP-GalNAc and CMP-Neu5Ac and donor substrates used in the production of glycoproteins and glycolipids. Growing evidence demonstrates that alteration of the pool of activated substrates might lead to different glycosylation and cell signaling. It is already well established that aberrant glycosylation can modulate tumor growth and malignant transformation in different cancer types. Therefore, biosynthetic machinery involved in the assembly of aberrant glycans are becoming prominent targets for anti-tumor drugs. This review describes three classes of glycosylation, O-GlcNAcylation, N-linked, and mucin type O-linked glycosylation, involved in tumor progression, their biosynthesis and highlights the available inhibitors as potential anti-tumor drugs.
Keywords: N-linked glycan; O-linked glycan; cancer; glycans; glycoconjugate; glycosyltransferases; hexosamine biosynthetic pathway; inhibitors.
Figures
Similar articles
-
Hexosamine Biosynthetic Pathway and Glycosylation Regulate Cell Migration in Melanoma Cells.Front Oncol. 2019 Mar 5;9:116. doi: 10.3389/fonc.2019.00116. eCollection 2019. Front Oncol. 2019. PMID: 30891426 Free PMC article.
-
Cellular glycosylation senses metabolic changes and modulates cell plasticity during epithelial to mesenchymal transition.Dev Dyn. 2018 Mar;247(3):481-491. doi: 10.1002/dvdy.24553. Epub 2017 Aug 18. Dev Dyn. 2018. PMID: 28722313 Review.
-
Disruption of O-GlcNAc Cycling in C. elegans Perturbs Nucleotide Sugar Pools and Complex Glycans.Front Endocrinol (Lausanne). 2014 Nov 24;5:197. doi: 10.3389/fendo.2014.00197. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 25505447 Free PMC article.
-
The Nutrient-Sensing Hexosamine Biosynthetic Pathway as the Hub of Cancer Metabolic Rewiring.Cells. 2018 Jun 2;7(6):53. doi: 10.3390/cells7060053. Cells. 2018. PMID: 29865240 Free PMC article. Review.
-
Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer.BMC Biol. 2019 Jul 4;17(1):52. doi: 10.1186/s12915-019-0671-3. BMC Biol. 2019. PMID: 31272438 Free PMC article. Review.
Cited by
-
Blocking ATM Attenuates SKOV3 Cell Proliferation and Migration by Disturbing OGT/OGA Expression via hsa-miR-542-5p.Front Oncol. 2022 Jun 20;12:839508. doi: 10.3389/fonc.2022.839508. eCollection 2022. Front Oncol. 2022. PMID: 35795059 Free PMC article.
-
Molecular Modeling Insights into the Structure and Behavior of Integrins: A Review.Cells. 2023 Jan 14;12(2):324. doi: 10.3390/cells12020324. Cells. 2023. PMID: 36672259 Free PMC article. Review.
-
The role of glycosyltransferase enzyme GCNT3 in colon and ovarian cancer prognosis and chemoresistance.Sci Rep. 2018 May 31;8(1):8485. doi: 10.1038/s41598-018-26468-4. Sci Rep. 2018. PMID: 29855486 Free PMC article.
-
High expression of GFAT1 predicts poor prognosis in patients with pancreatic cancer.Sci Rep. 2016 Dec 20;6:39044. doi: 10.1038/srep39044. Sci Rep. 2016. PMID: 27996048 Free PMC article. Clinical Trial.
-
Prognostic relevance of the hexosamine biosynthesis pathway activation in leiomyosarcoma.NPJ Genom Med. 2021 May 3;6(1):30. doi: 10.1038/s41525-021-00193-w. NPJ Genom Med. 2021. PMID: 33941787 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

