Improving the Post-Stroke Therapeutic Potency of Mesenchymal Multipotent Stromal Cells by Cocultivation With Cortical Neurons: The Role of Crosstalk Between Cells
- PMID: 26160961
- PMCID: PMC4542870
- DOI: 10.5966/sctm.2015-0010
Improving the Post-Stroke Therapeutic Potency of Mesenchymal Multipotent Stromal Cells by Cocultivation With Cortical Neurons: The Role of Crosstalk Between Cells
Abstract
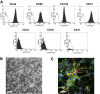
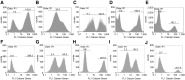
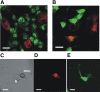
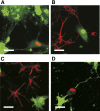
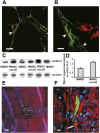
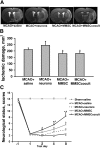
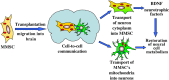
The goal of the present study was to maximally alleviate the negative impact of stroke by increasing the therapeutic potency of injected mesenchymal multipotent stromal cells (MMSCs). To pursue this goal, the intercellular communications of MMSCs and neuronal cells were studied in vitro. As a result of cocultivation of MMSCs and rat cortical neurons, we proved the existence of intercellular contacts providing transfer of cellular contents from one cell to another. We present evidence of intercellular exchange with fluorescent probes specifically occupied by cytosol with preferential transfer from neurons toward MMSCs. In contrast, we observed a reversed transfer of mitochondria (from MMSCs to neural cells). Intravenous injection of MMSCs in a postischemic period alleviated the pathological indexes of a stroke, expressed as a lower infarct volume in the brain and partial restoration of neurological status. Also, MMSCs after cocultivation with neurons demonstrated more profound neuroprotective effects than did unprimed MMSCs. The production of the brain-derived neurotrophic factor was slightly increased in MMSCs, and the factor itself was redistributed in these cells after cocultivation. The level of Miro1 responsible for intercellular traffic of mitochondria was increased in MMSCs after cocultivation. We conclude that the exchange by cellular compartments between neural and stem cells improves MMSCs' protective abilities for better rehabilitation after stroke. This could be used as an approach to enhance the therapeutic benefits of stem cell therapy to the damaged brain.
Significance: The idea of priming stem cells before practical use for clinical purposes was applied. Thus, cells were preconditioned by coculturing them with the targeted cells (i.e., neurons for the treatment of brain pathological features) before the transfusion of stem cells to the organism. Such priming improved the capacity of stem cells to treat stroke. Some additional minimal study will be required to develop a detailed protocol for coculturing followed by cell separation.
Keywords: Astrocytes; Intercellular communication; Ischemia; Mitochondria; Neurons; Stem cell therapy; Stroke.
©AlphaMed Press.
Figures
Similar articles
-
Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery.Molecules. 2018 Mar 19;23(3):687. doi: 10.3390/molecules23030687. Molecules. 2018. PMID: 29562677 Free PMC article.
-
Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1.J Cereb Blood Flow Metab. 2021 Apr;41(4):761-770. doi: 10.1177/0271678X20928147. Epub 2020 Jun 5. J Cereb Blood Flow Metab. 2021. PMID: 32501156 Free PMC article.
-
Therapeutic Potential of a Combination of Electroacupuncture and TrkB-Expressing Mesenchymal Stem Cells for Ischemic Stroke.Mol Neurobiol. 2019 Jan;56(1):157-173. doi: 10.1007/s12035-018-1067-z. Epub 2018 Apr 22. Mol Neurobiol. 2019. PMID: 29682700
-
Bone Marrow-Derived NCS-01 Cells Advance a Novel Cell-Based Therapy for Stroke.Int J Mol Sci. 2020 Apr 19;21(8):2845. doi: 10.3390/ijms21082845. Int J Mol Sci. 2020. PMID: 32325813 Free PMC article. Review.
-
Recent Progress in Therapeutic Strategies for Ischemic Stroke.Cell Transplant. 2016;25(5):893-8. doi: 10.3727/096368916X690548. Epub 2016 Jan 18. Cell Transplant. 2016. PMID: 26786838 Review.
Cited by
-
Mesenchymal stromal cell mitochondrial transfer to human induced T-regulatory cells mediates FOXP3 stability.Sci Rep. 2021 May 21;11(1):10676. doi: 10.1038/s41598-021-90115-8. Sci Rep. 2021. PMID: 34021231 Free PMC article.
-
Communication in the Cancer Microenvironment as a Target for Therapeutic Interventions.Cancers (Basel). 2020 May 14;12(5):1232. doi: 10.3390/cancers12051232. Cancers (Basel). 2020. PMID: 32422889 Free PMC article. Review.
-
Endothelial progenitor cells transplantation attenuated blood-brain barrier damage after ischemia in diabetic mice via HIF-1α.Stem Cell Res Ther. 2017 Jul 11;8(1):163. doi: 10.1186/s13287-017-0605-3. Stem Cell Res Ther. 2017. PMID: 28697748 Free PMC article.
-
Intercellular mitochondrial transfer as a means of tissue revitalization.Signal Transduct Target Ther. 2021 Feb 16;6(1):65. doi: 10.1038/s41392-020-00440-z. Signal Transduct Target Ther. 2021. PMID: 33589598 Free PMC article. Review.
-
Mesenchymal Stem Cell-Mediated Mitochondrial Transfer: a Therapeutic Approach for Ischemic Stroke.Transl Stroke Res. 2021 Apr;12(2):212-229. doi: 10.1007/s12975-020-00853-6. Epub 2020 Sep 25. Transl Stroke Res. 2021. PMID: 32975692 Review.
References
-
- Röther J. Neuroprotection does not work! Stroke. 2008;39:523–524. - PubMed
-
- Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. - PubMed
-
- Barry FP, Murphy JM, English K, et al. Immunogenicity of adult mesenchymal stem cells: Lessons from the fetal allograft. Stem Cells Dev. 2005;14:252–265. - PubMed
-
- Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

