Ly6Chigh Monocytes Protect against Kidney Damage during Sepsis via a CX3CR1-Dependent Adhesion Mechanism
- PMID: 26160897
- PMCID: PMC4769199
- DOI: 10.1681/ASN.2015010009
Ly6Chigh Monocytes Protect against Kidney Damage during Sepsis via a CX3CR1-Dependent Adhesion Mechanism
Abstract
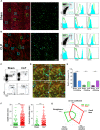
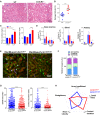
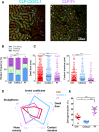
Monocytes have a crucial role in both proinflammatory and anti-inflammatory phenomena occurring during sepsis. Monocyte recruitment and activation are orchestrated by the chemokine receptors CX3CR1 and CCR2 and their cognate ligands. However, little is known about the roles of these cells and chemokines during the acute phase of inflammation in sepsis. Using intravital microscopy in a murine model of polymicrobial sepsis, we showed that inflammatory Ly6C(high) monocytes infiltrated kidneys, exhibited altered motility, and adhered strongly to the renal vascular wall in a chemokine receptor CX3CR1-dependent manner. Adoptive transfer of Cx3cr1-proficient monocyte-enriched bone marrow cells into septic Cx3cr1-depleted mice prevented kidney damage and promoted mouse survival. Modulation of CX3CR1 activation in septic mice controlled monocyte adhesion, regulated proinflammatory and anti-inflammatory cytokine expression, and was associated with the extent of kidney lesions such that the number of lesions decreased when CX3CR1 activity increased. Consistent with these results, the pro-adhesive I249 CX3CR1 allele in humans was associated with a lower incidence of AKI in patients with sepsis. These data show that inflammatory monocytes have a protective effect during sepsis via a CX3CR1-dependent adhesion mechanism. This receptor might be a new therapeutic target for kidney injury during sepsis.
Keywords: chemokine receptor; immunology; kidney dysfunction.
Copyright © 2016 by the American Society of Nephrology.
Figures

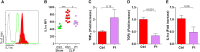
Similar articles
-
CX3CR1 reduces Ly6Chigh-monocyte motility within and release from the bone marrow after chemotherapy in mice.Blood. 2013 Aug 1;122(5):674-83. doi: 10.1182/blood-2013-01-480749. Epub 2013 Jun 17. Blood. 2013. PMID: 23775714
-
The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury.Kidney Int. 2008 Dec;74(12):1526-37. doi: 10.1038/ki.2008.500. Epub 2008 Oct 8. Kidney Int. 2008. PMID: 18843253 Free PMC article.
-
CX3CL1-CX3CR1 Interaction Increases the Population of Ly6C(-)CX3CR1(hi) Macrophages Contributing to Unilateral Ureteral Obstruction-Induced Fibrosis.J Immunol. 2015 Sep 15;195(6):2797-805. doi: 10.4049/jimmunol.1403209. Epub 2015 Aug 7. J Immunol. 2015. PMID: 26254342
-
Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis.Arterioscler Thromb Vasc Biol. 2009 Oct;29(10):1412-8. doi: 10.1161/ATVBAHA.108.180505. Arterioscler Thromb Vasc Biol. 2009. PMID: 19759373 Free PMC article. Review.
-
Nonclassical patrolling monocyte function in the vasculature.Arterioscler Thromb Vasc Biol. 2015 Jun;35(6):1306-16. doi: 10.1161/ATVBAHA.114.304650. Epub 2015 Apr 2. Arterioscler Thromb Vasc Biol. 2015. PMID: 25838429 Free PMC article. Review.
Cited by
-
A double-edged sword: interactions of CX3CL1/CX3CR1 and gut microbiota in systemic lupus erythematosus.Front Immunol. 2024 Jan 17;14:1330500. doi: 10.3389/fimmu.2023.1330500. eCollection 2023. Front Immunol. 2024. PMID: 38299151 Free PMC article. Review.
-
Occurrences and Functions of Ly6Chi and Ly6Clo Macrophages in Health and Disease.Front Immunol. 2022 May 30;13:901672. doi: 10.3389/fimmu.2022.901672. eCollection 2022. Front Immunol. 2022. PMID: 35707538 Free PMC article. Review.
-
When the Renal (Function) Begins to Fall: A Mini-Review of Acute Kidney Injury Related to Acute Respiratory Distress Syndrome in Critically Ill Patients.Front Nephrol. 2022 Apr 21;2:877529. doi: 10.3389/fneph.2022.877529. eCollection 2022. Front Nephrol. 2022. PMID: 37675005 Free PMC article. Review.
-
Immune cell behaviour and dynamics in the kidney - insights from in vivo imaging.Nat Rev Nephrol. 2022 Jan;18(1):22-37. doi: 10.1038/s41581-021-00481-9. Epub 2021 Sep 23. Nat Rev Nephrol. 2022. PMID: 34556836 Review.
-
Discrepant Phenotyping of Monocytes Based on CX3CR1 and CCR2 Using Fluorescent Reporters and Antibodies.Cells. 2024 May 10;13(10):819. doi: 10.3390/cells13100819. Cells. 2024. PMID: 38786041 Free PMC article.
References
-
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ, The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine : Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101: 1644–1655, 1992 - PubMed
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR: Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001 - PubMed
-
- Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D, Sepsis Occurrence in Acutely Ill Patients Investigators : Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med 34: 344–353, 2006 - PubMed
-
- Angus DC, van der Poll T: Severe sepsis and septic shock. N Engl J Med 369: 840–851, 2013 - PubMed
-
- Cohen J: The immunopathogenesis of sepsis. Nature 420: 885–891, 2002 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

