The multifaceted biology of plasmacytoid dendritic cells
- PMID: 26160613
- PMCID: PMC4808588
- DOI: 10.1038/nri3865
The multifaceted biology of plasmacytoid dendritic cells
Abstract
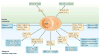
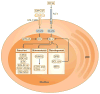
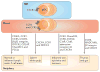
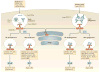
Plasmacytoid dendritic cells (pDCs) are a unique DC subset that specializes in the production of type I interferons (IFNs). pDCs promote antiviral immune responses and have been implicated in the pathogenesis of autoimmune diseases that are characterized by a type I IFN signature. However, pDCs can also induce tolerogenic immune responses. In this Review, we summarize recent progress in the field of pDC biology, focusing on the molecular mechanisms that regulate the development and functions of pDCs, the pathways involved in their sensing of pathogens and endogenous nucleic acids, their functions at mucosal sites, and their roles in infection, autoimmunity and cancer.
Figures
Similar articles
-
Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance.Immunol Rev. 2010 Mar;234(1):142-62. doi: 10.1111/j.0105-2896.2009.00881.x. Immunol Rev. 2010. PMID: 20193017 Free PMC article. Review.
-
Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases.Nat Rev Immunol. 2008 Aug;8(8):594-606. doi: 10.1038/nri2358. Nat Rev Immunol. 2008. PMID: 18641647 Review.
-
Plasmacytoid dendritic cells in antiviral immunity and autoimmunity.Sci China Life Sci. 2010 Feb;53(2):172-82. doi: 10.1007/s11427-010-0045-0. Epub 2010 Mar 7. Sci China Life Sci. 2010. PMID: 20596824 Free PMC article. Review.
-
Type I Interferon Production of Plasmacytoid Dendritic Cells under Control.Int J Mol Sci. 2021 Apr 18;22(8):4190. doi: 10.3390/ijms22084190. Int J Mol Sci. 2021. PMID: 33919546 Free PMC article. Review.
-
Plasmacytoid Dendritic Cells: Development, Regulation, and Function.Immunity. 2019 Jan 15;50(1):37-50. doi: 10.1016/j.immuni.2018.12.027. Immunity. 2019. PMID: 30650380 Free PMC article. Review.
Cited by
-
Engineering dendritic cell biomimetic membrane as a delivery system for tumor targeted therapy.J Nanobiotechnology. 2024 Oct 27;22(1):663. doi: 10.1186/s12951-024-02913-7. J Nanobiotechnology. 2024. PMID: 39465376 Free PMC article. Review.
-
The TNFSF Members APRIL and BAFF and Their Receptors TACI, BCMA, and BAFFR in Oncology, With a Special Focus in Breast Cancer.Front Oncol. 2020 Jun 16;10:827. doi: 10.3389/fonc.2020.00827. eCollection 2020. Front Oncol. 2020. PMID: 32612943 Free PMC article.
-
Innate Immune Responses to Highly Pathogenic Coronaviruses and Other Significant Respiratory Viral Infections.Front Immunol. 2020 Aug 18;11:1979. doi: 10.3389/fimmu.2020.01979. eCollection 2020. Front Immunol. 2020. PMID: 32973803 Free PMC article. Review.
-
Effects of Heat-Killed Lacticaseibacillus paracasei MCC1849 on Immune Parameters in Healthy Adults-A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study.Nutrients. 2024 Jan 9;16(2):216. doi: 10.3390/nu16020216. Nutrients. 2024. PMID: 38257109 Free PMC article. Clinical Trial.
-
Gut-Joint Axis: Impact of Bifidobacterial Cell Wall Lipoproteins on Arthritis Development.Nutrients. 2023 Nov 21;15(23):4861. doi: 10.3390/nu15234861. Nutrients. 2023. PMID: 38068720 Free PMC article.
References
-
- Lennert K, Remmele W. Karyometric research on lymph node cells in man. I. Germinoblasts, lymphoblasts & lymphocytes. Acta Haematol. 1958;19:99–113. - PubMed
-
- Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. - PubMed
-
- Cella M, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. - PubMed
-
- Howell DM, Feldman M, Siegal FP, Pettera L, Fitzgerald-Bocarsly P. Peripheral blood of AIDS patients contains cells capable of providing accessory function for the natural killer cell-mediated, lysis of herpes simplex virus-infected targets despite low interferon-alpha production. J Acquir Immune Defic Syndr. 1993;6:15–23. - PubMed
-
- Perussia B, Fanning V, Trinchieri G. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat Immun Cell Growth Regul. 1985;4:120–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

