RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation
- PMID: 26159994
- PMCID: PMC4511210
- DOI: 10.1101/gad.262766.115
RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation
Abstract
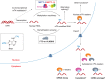
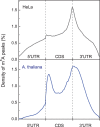
N(6)-methyladenosine (m(6)A) is the most prevalent and internal modification that occurs in the messenger RNAs (mRNA) of most eukaryotes, although its functional relevance remained a mystery for decades. This modification is installed by the m(6)A methylation "writers" and can be reversed by demethylases that serve as "erasers." In this review, we mainly summarize recent progress in the study of the m(6)A mRNA methylation machineries across eukaryotes and discuss their newly uncovered biological functions. The broad roles of m(6)A in regulating cell fates and embryonic development highlight the existence of another layer of epigenetic regulation at the RNA level, where mRNA is subjected to chemical modifications that affect protein expression.
Keywords: METTL3–METTL14; N6-methyladenosine; RNA demethylase; m6A methyltransferase; mRNA methylation; post-transcriptional regulation.
© 2015 Yue et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


Similar articles
-
Epigenetic regulation of mRNA N6-methyladenosine modifications in mammalian gametogenesis.Mol Hum Reprod. 2021 May 8;27(5):gaab025. doi: 10.1093/molehr/gaab025. Mol Hum Reprod. 2021. PMID: 33823008 Review.
-
Function and evolution of RNA N6-methyladenosine modification.Int J Biol Sci. 2020 Apr 15;16(11):1929-1940. doi: 10.7150/ijbs.45231. eCollection 2020. Int J Biol Sci. 2020. PMID: 32398960 Free PMC article. Review.
-
m6 A RNA methylation: from mechanisms to therapeutic potential.EMBO J. 2021 Feb 1;40(3):e105977. doi: 10.15252/embj.2020105977. Epub 2021 Jan 20. EMBO J. 2021. PMID: 33470439 Free PMC article. Review.
-
N (6)-Methyladenosine (m(6)A) Methylation in mRNA with A Dynamic and Reversible Epigenetic Modification.Mol Biotechnol. 2016 Jul;58(7):450-9. doi: 10.1007/s12033-016-9947-9. Mol Biotechnol. 2016. PMID: 27179969 Review.
-
RNA N6-methyladenosine methylation and skin diseases.Autoimmunity. 2023 Dec;56(1):2167983. doi: 10.1080/08916934.2023.2167983. Autoimmunity. 2023. PMID: 36708146 Review.
Cited by
-
The Significance of N6-Methyladenosine RNA Methylation in Regulating the Hepatitis B Virus Life Cycle.J Microbiol Biotechnol. 2024 Feb 28;34(2):233-239. doi: 10.4014/jmb.2309.09013. Epub 2023 Oct 31. J Microbiol Biotechnol. 2024. PMID: 37942519 Free PMC article. Review.
-
The role of N6-methyladenosine modification in the life cycle and disease pathogenesis of hepatitis B and C viruses.Exp Mol Med. 2021 Mar;53(3):339-345. doi: 10.1038/s12276-021-00581-3. Epub 2021 Mar 19. Exp Mol Med. 2021. PMID: 33742132 Free PMC article. Review.
-
Post-transcriptional control of T-cell cytokine production: Implications for cancer therapy.Immunology. 2021 Sep;164(1):57-72. doi: 10.1111/imm.13339. Epub 2021 May 10. Immunology. 2021. PMID: 33884612 Free PMC article. Review.
-
Loss-of-Function Genetic Screening Identifies Aldolase A as an Essential Driver for Liver Cancer Cell Growth Under Hypoxia.Hepatology. 2021 Sep;74(3):1461-1479. doi: 10.1002/hep.31846. Hepatology. 2021. PMID: 33813748 Free PMC article.
-
Modulation of DNA/RNA Methylation by Small-Molecule Modulators and Their Implications in Cancer.Subcell Biochem. 2022;100:557-579. doi: 10.1007/978-3-031-07634-3_17. Subcell Biochem. 2022. PMID: 36301506
References
-
- Agris PF, Vendeix FAP, Graham WD. 2007. tRNA's wobble decoding of the genome: 40 years of modification. J Mol Biol 366: 1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
