Kv1.3 contains an alternative C-terminal ER exit motif and is recruited into COPII vesicles by Sec24a
- PMID: 26156069
- PMCID: PMC4497498
- DOI: 10.1186/s12858-015-0045-6
Kv1.3 contains an alternative C-terminal ER exit motif and is recruited into COPII vesicles by Sec24a
Abstract
Background: Potassium channels play a fundamental role in resetting the resting membrane potential of excitable cells. Determining the intracellular trafficking and localization mechanisms of potassium channels provides a platform to fully characterize their maturation and functionality. Previous investigations have discovered residues or motifs that exist in their primary structure, which directly promote anterograde trafficking of nascent potassium channels. Recently, a non-conical di-acidic motif (E483/484) has been discovered in the C-terminus of the mammalian homologue of the Shaker voltage-gated potassium channel subfamily member 3 (Kv1.3), and was shown to disrupt the anterograde trafficking of Kv1.3.
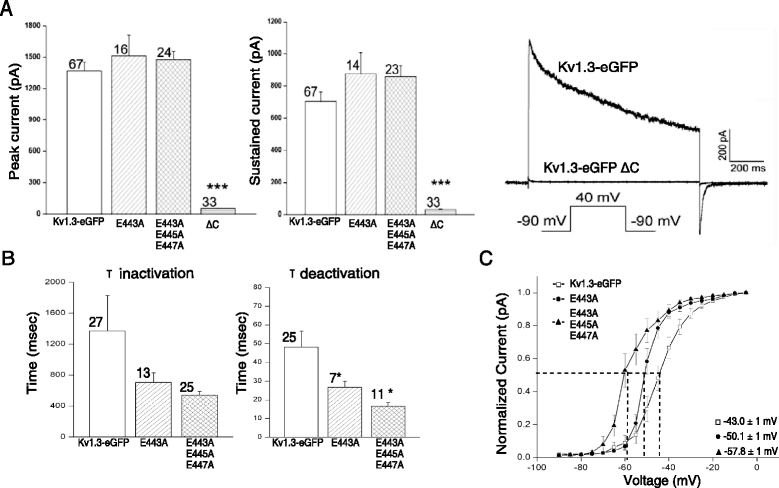
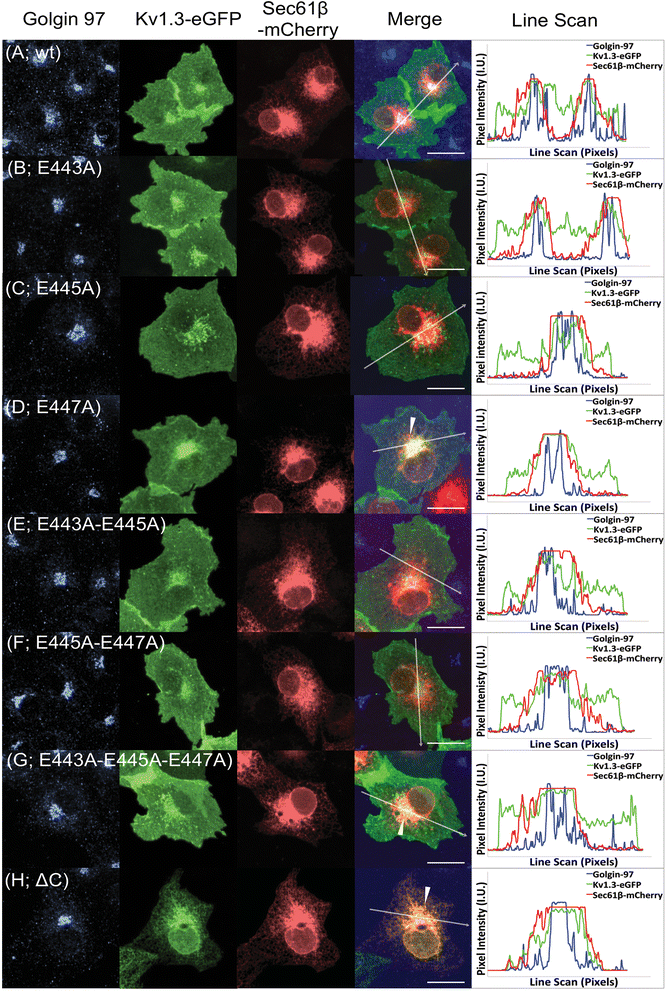
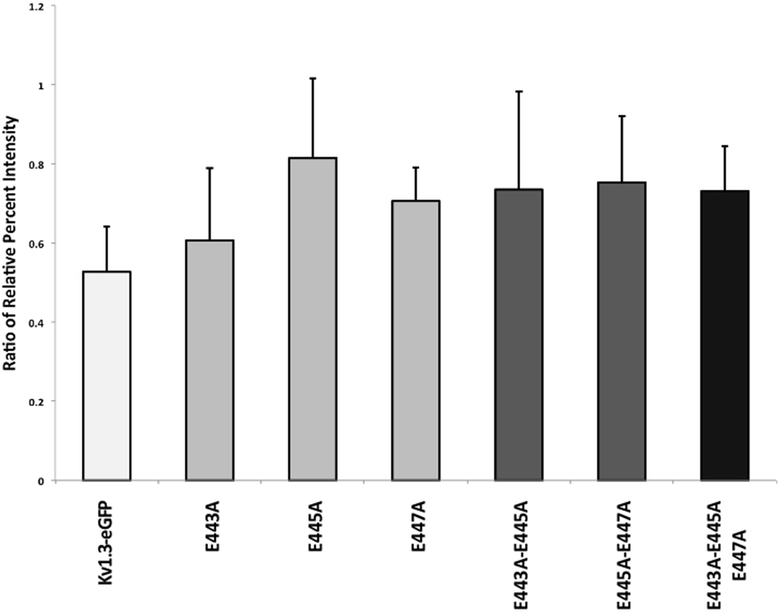
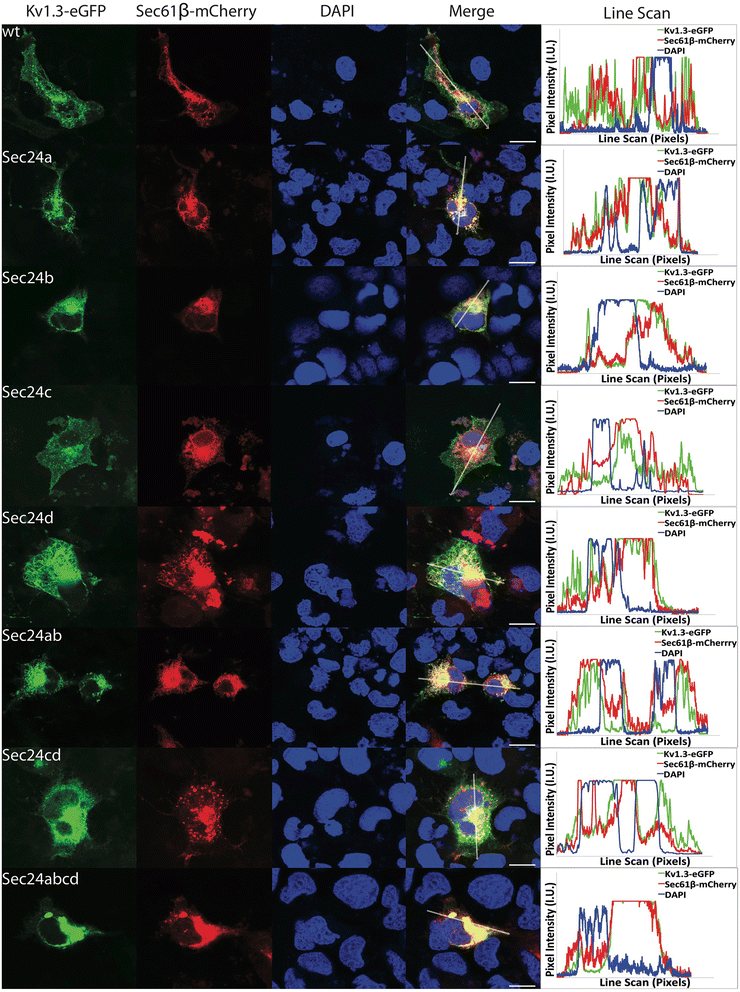
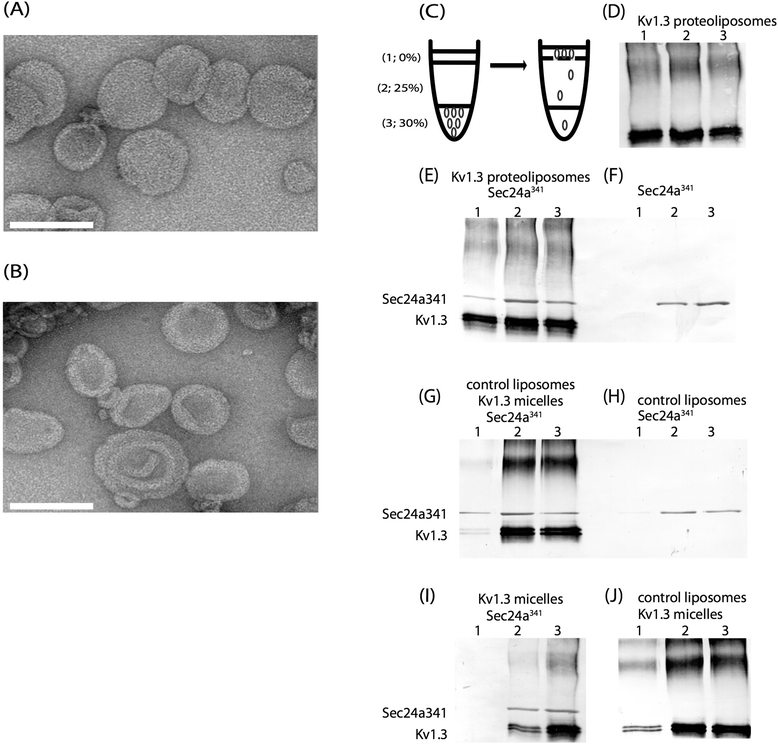
Results: We have further investigated the intracellular trafficking requirements of Kv1.3 both in vivo and in vitro. First, three alternative C-terminal acidic residues, E443, E445, E447 were probed for their involvement within the early secretory pathway of Kv1.3. Single point (E443A, E445A, and E447A) and double point (E443A-E445A, E445A-E447A) mutations exhibited no significant changes in their endoplasmic reticulum (ER) retention. The triple point mutant E443A-E445A-E447A displayed a modest ER retention while deletion of the C-terminus showed dramatic ER retention. Second, we demonstrate in vivo the requirement for the Sec24a isoform to confer anterograde trafficking using a siRNA knockdown assay. Third, we show in vitro the association of recombinantly expressed Kv1.3 and Sec24a proteins.
Conclusion: These results expand upon previous studies aimed at deciphering the Kv1.3 secretory trafficking mechanisms and further show in vitro evidence of the association between Kv1.3 and the COPII cargo adaptor subunit isoform Sec24a.
Figures
Similar articles
-
The C-terminal domain of Kv1.3 regulates functional interactions with the KCNE4 subunit.J Cell Sci. 2016 Nov 15;129(22):4265-4277. doi: 10.1242/jcs.191650. Epub 2016 Oct 6. J Cell Sci. 2016. PMID: 27802162 Free PMC article.
-
A non-canonical di-acidic signal at the C-terminus of Kv1.3 determines anterograde trafficking and surface expression.J Cell Sci. 2013 Dec 15;126(Pt 24):5681-91. doi: 10.1242/jcs.134825. Epub 2013 Oct 21. J Cell Sci. 2013. PMID: 24144698
-
SEC24A deficiency lowers plasma cholesterol through reduced PCSK9 secretion.Elife. 2013 Apr 9;2:e00444. doi: 10.7554/eLife.00444. Elife. 2013. PMID: 23580231 Free PMC article.
-
ER-to-Golgi transport: form and formation of vesicular and tubular carriers.Biochim Biophys Acta. 2005 Jul 10;1744(3):304-15. doi: 10.1016/j.bbamcr.2005.03.003. Epub 2005 Mar 23. Biochim Biophys Acta. 2005. PMID: 15979504 Review.
-
COPII-coated vesicles: flexible enough for large cargo?Curr Opin Cell Biol. 2005 Aug;17(4):345-52. doi: 10.1016/j.ceb.2005.06.004. Curr Opin Cell Biol. 2005. PMID: 15975775 Review.
Cited by
-
Therapeutic Antibodies Targeting Potassium Ion Channels.Handb Exp Pharmacol. 2021;267:507-545. doi: 10.1007/164_2021_464. Handb Exp Pharmacol. 2021. PMID: 33963460
-
The C-terminal domain of Kv1.3 regulates functional interactions with the KCNE4 subunit.J Cell Sci. 2016 Nov 15;129(22):4265-4277. doi: 10.1242/jcs.191650. Epub 2016 Oct 6. J Cell Sci. 2016. PMID: 27802162 Free PMC article.
-
The Potassium Channel Odyssey: Mechanisms of Traffic and Membrane Arrangement.Int J Mol Sci. 2019 Feb 9;20(3):734. doi: 10.3390/ijms20030734. Int J Mol Sci. 2019. PMID: 30744118 Free PMC article. Review.
-
Combining mKate2-Kv1.3 Channel and Atto488-Hongotoxin for the Studies of Peptide Pore Blockers on Living Eukaryotic Cells.Toxins (Basel). 2022 Dec 5;14(12):858. doi: 10.3390/toxins14120858. Toxins (Basel). 2022. PMID: 36548755 Free PMC article.
-
Modulating the Excitability of Olfactory Output Neurons Affects Whole-Body Metabolism.J Neurosci. 2022 Jul 27;42(30):5966-5990. doi: 10.1523/JNEUROSCI.0190-22.2022. Epub 2022 Jun 16. J Neurosci. 2022. PMID: 35710623 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

