TCR signaling intensity controls CD8+ T cell responsiveness to TGF-β
- PMID: 26153417
- PMCID: PMC4600064
- DOI: 10.1189/jlb.2HIMA1214-578R
TCR signaling intensity controls CD8+ T cell responsiveness to TGF-β
Abstract
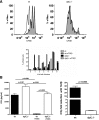
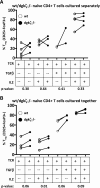
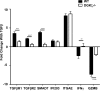
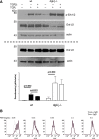
DGK-ζ is a negative regulator of TCR signaling that causes degradation of the second messenger DAG, terminating DAG-mediated activation of Ras and PKCθ. Cytotoxic T cells deficient in DGK-ζ demonstrate enhanced effector functions in vitro and antitumor activity in vivo, perhaps because of insensitivity to inhibitory cytokines. We sought to determine whether the enhanced responsiveness of DGK-ζ-deficient T cells renders them insensitive to the inhibitory cytokine TGF-β and to determine how the loss of DGK-ζ facilitates this insensitivity. We identified decreased transcriptional and functional responses to TGF-β in CD8(+) DGK-ζ(-/-) T cells but preserved TGF-β-mediated conversion of naïve DGK-ζ(-/-) CD4(+) T cells to a regulatory T cell phenotype. Decreased CD8(+) T cell responsiveness to TGF-β did not result from impaired canonical TGF-β signal transduction, because similar levels of TGF-β-R and intracellular Smad components were identified in WT and DGK-ζ(-/-) CD8(+) T cells, and TGF-β-mediated activation of Smad2 was unchanged. Instead, an enhanced TCR signal strength was responsible for TGF-β insensitivity, because (i) loss of DGK-ζ conferred resistance to TGF-β-mediated inhibition of Erk phosphorylation, (ii) TGF-β insensitivity could be recapitulated by exogenous addition of the DAG analog PMA, and (iii) TGF-β sensitivity could be observed in DGK-ζ-deficient T cells at limiting dilutions of TCR stimulation. These data indicate that enhanced TCR signal transduction in the absence of DGK-ζ makes T cells relatively insensitive to TGF-β, in a manner independent of Smads, a finding with practical implications in the development of immunotherapies that target TGF-β.
Keywords: Diacylglycerol; Smad2; diacylglycerol kinase ζ.
© Society for Leukocyte Biology.
Figures


Comment in
-
Editorial: Tweaking T cell receptor signaling thresholds through DAG: the role of diacylglycerol kinase ζ in T cell responses to TGF-β.J Leukoc Biol. 2015 Nov;98(5):685-7. doi: 10.1189/jlb.2CE0615-278. J Leukoc Biol. 2015. PMID: 26525843 No abstract available.
Similar articles
-
Editorial: Tweaking T cell receptor signaling thresholds through DAG: the role of diacylglycerol kinase ζ in T cell responses to TGF-β.J Leukoc Biol. 2015 Nov;98(5):685-7. doi: 10.1189/jlb.2CE0615-278. J Leukoc Biol. 2015. PMID: 26525843 No abstract available.
-
CRISPR/Cas9-Mediated Knockout of DGK Improves Antitumor Activities of Human T Cells.Cancer Res. 2018 Aug 15;78(16):4692-4703. doi: 10.1158/0008-5472.CAN-18-0030. Epub 2018 Jul 2. Cancer Res. 2018. PMID: 29967261
-
The ζ isoform of diacylglycerol kinase plays a predominant role in regulatory T cell development and TCR-mediated ras signaling.Sci Signal. 2013 Nov 26;6(303):ra102. doi: 10.1126/scisignal.2004373. Sci Signal. 2013. PMID: 24280043 Free PMC article.
-
Role of diacylglycerol kinases in T cell development and function.Crit Rev Immunol. 2013;33(2):97-118. doi: 10.1615/critrevimmunol.2013006696. Crit Rev Immunol. 2013. PMID: 23582058 Free PMC article. Review.
-
Redundant and specialized roles for diacylglycerol kinases α and ζ in the control of T cell functions.Sci Signal. 2015 Apr 28;8(374):re6. doi: 10.1126/scisignal.aaa0974. Sci Signal. 2015. PMID: 25921290 Review.
Cited by
-
The adhesion molecule PECAM-1 enhances the TGF-β-mediated inhibition of T cell function.Sci Signal. 2016 Mar 8;9(418):ra27. doi: 10.1126/scisignal.aad1242. Sci Signal. 2016. PMID: 26956486 Free PMC article.
-
TGFβ limits Myc-dependent TCR-induced metabolic reprogramming in CD8+ T cells.Front Immunol. 2022 Jul 26;13:913184. doi: 10.3389/fimmu.2022.913184. eCollection 2022. Front Immunol. 2022. PMID: 35958566 Free PMC article.
-
Diacylglycerol kinase ζ (DGKζ) and Casitas b-lineage proto-oncogene b-deficient mice have similar functional outcomes in T cells but DGKζ-deficient mice have increased T cell activation and tumor clearance.Immunohorizons. 2018 Apr 1;2(4):107-118. doi: 10.4049/immunohorizons.1700055. Immunohorizons. 2018. PMID: 30027154 Free PMC article.
-
DGK-α: A Checkpoint in Cancer-Mediated Immuno-Inhibition and Target for Immunotherapy.Front Cell Dev Biol. 2017 Mar 3;5:16. doi: 10.3389/fcell.2017.00016. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 28316970 Free PMC article.
-
Age-Related Dynamics of Lung-Resident Memory CD8+ T Cells in the Age of COVID-19.Front Immunol. 2021 Mar 29;12:636118. doi: 10.3389/fimmu.2021.636118. eCollection 2021. Front Immunol. 2021. PMID: 33854506 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

