The tortoise or the hare? Impacts of within-host dynamics on transmission success of arthropod-borne viruses
- PMID: 26150665
- PMCID: PMC4528497
- DOI: 10.1098/rstb.2014.0299
The tortoise or the hare? Impacts of within-host dynamics on transmission success of arthropod-borne viruses
Abstract
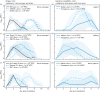
Arthropod-borne viruses (arboviruses) are maintained in a cycle of alternating transmission between vertebrate hosts and arthropod vectors. Arboviruses possess RNA genomes capable of rapid diversification and adaptation, and the between-host trade-offs inherent to host alternation impose well-documented constraints on arbovirus evolution. Here, we investigate the less well-studied within-host trade-offs that shape arbovirus replication dynamics and transmission. Arboviruses generally establish lifelong infection in vectors but transient infection of variable magnitude (i.e. peak virus concentration) and duration in vertebrate hosts. In the majority of experimental infections of vertebrate hosts, both the magnitude and duration of arbovirus replication depended upon the dose of virus administered, with increasing dose resulting in greater magnitude but shorter duration of viraemia. This pattern suggests that the vertebrate immune response imposes a trade-off between the height and breadth of the virus replication curve. To investigate the impact of this trade-off on transmission, we used a simple modelling approach to contrast the effect of 'tortoise' (low magnitude, long duration viraemia) and 'hare' (high magnitude, short duration viraemia) arbovirus replication strategies on transmission. This model revealed that, counter to previous theory, arboviruses that adopt a tortoise strategy have higher rates of persistence in both host and vector populations.
Keywords: arthropod-borne virus; dynamics; host; trade-off; transmission; vector.
© 2015 The Author(s) Published by the Royal Society. All rights reserved.
Figures

Similar articles
-
Arbovirus-mosquito interactions: RNAi pathway.Curr Opin Virol. 2015 Dec;15:119-26. doi: 10.1016/j.coviro.2015.10.001. Epub 2015 Dec 6. Curr Opin Virol. 2015. PMID: 26629932 Free PMC article. Review.
-
Tissue Barriers to Arbovirus Infection in Mosquitoes.Viruses. 2015 Jul 8;7(7):3741-67. doi: 10.3390/v7072795. Viruses. 2015. PMID: 26184281 Free PMC article. Review.
-
Evolutionary influences in arboviral disease.Curr Top Microbiol Immunol. 2006;299:285-314. doi: 10.1007/3-540-26397-7_10. Curr Top Microbiol Immunol. 2006. PMID: 16568903 Free PMC article. Review.
-
Effects of Arbovirus Multi-Host Life Cycles on Dinucleotide and Codon Usage Patterns.Viruses. 2019 Jul 12;11(7):643. doi: 10.3390/v11070643. Viruses. 2019. PMID: 31336898 Free PMC article. Review.
-
Arboviruses: How Saliva Impacts the Journey from Vector to Host.Int J Mol Sci. 2021 Aug 25;22(17):9173. doi: 10.3390/ijms22179173. Int J Mol Sci. 2021. PMID: 34502092 Free PMC article. Review.
Cited by
-
Quantifying Rift Valley fever virus transmission efficiency in a lamb-mosquito-lamb model.Front Cell Infect Microbiol. 2023 Dec 18;13:1206089. doi: 10.3389/fcimb.2023.1206089. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38170150 Free PMC article.
-
Mosquito-Independent Transmission of West Nile virus in Farmed Saltwater Crocodiles (Crocodylus porosus).Viruses. 2020 Feb 11;12(2):198. doi: 10.3390/v12020198. Viruses. 2020. PMID: 32054016 Free PMC article.
-
Ecological processes underlying the emergence of novel enzootic cycles: Arboviruses in the neotropics as a case study.PLoS Negl Trop Dis. 2020 Aug 13;14(8):e0008338. doi: 10.1371/journal.pntd.0008338. eCollection 2020 Aug. PLoS Negl Trop Dis. 2020. PMID: 32790670 Free PMC article. Review.
-
Linked within-host and between-host models and data for infectious diseases: a systematic review.PeerJ. 2019 Jun 19;7:e7057. doi: 10.7717/peerj.7057. eCollection 2019. PeerJ. 2019. PMID: 31249734 Free PMC article.
-
The Vector - Host - Pathogen Interface: The Next Frontier in the Battle Against Mosquito-Borne Viral Diseases?Front Cell Infect Microbiol. 2020 Oct 16;10:564518. doi: 10.3389/fcimb.2020.564518. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33178624 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

