Antimicrobial activity of synthetic cationic peptides and lipopeptides derived from human lactoferricin against Pseudomonas aeruginosa planktonic cultures and biofilms
- PMID: 26149536
- PMCID: PMC4491869
- DOI: 10.1186/s12866-015-0473-x
Antimicrobial activity of synthetic cationic peptides and lipopeptides derived from human lactoferricin against Pseudomonas aeruginosa planktonic cultures and biofilms
Abstract
Background: Infections by Pseudomonas aeruginosa constitute a serious health threat because this pathogen -particularly when it forms biofilms - can acquire resistance to the majority of conventional antibiotics. This study evaluated the antimicrobial activity of synthetic peptides based on LF11, an 11-mer peptide derived from human lactoferricin against P. aeruginosa planktonic and biofilm-forming cells. We included in this analysis selected N-acylated derivatives of the peptides to analyze the effect of acylation in antimicrobial activity. To assess the efficacy of compounds against planktonic bacteria, microdilution assays to determine the minimal inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and time-kill studies were conducted. The anti-biofilm activity of the agents was assessed on biofilms grown under static (on microplates) and dynamic (in a CDC-reactor) flow regimes.
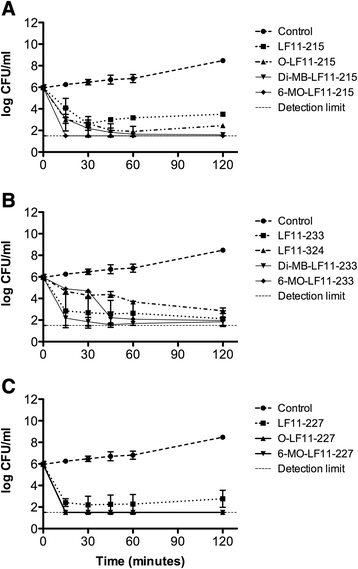
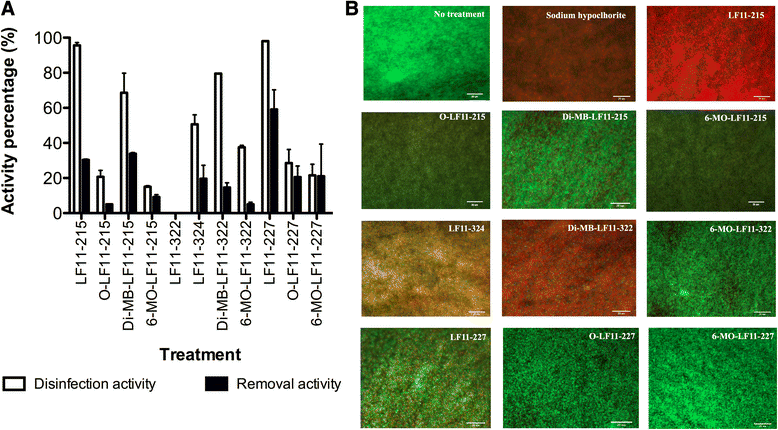
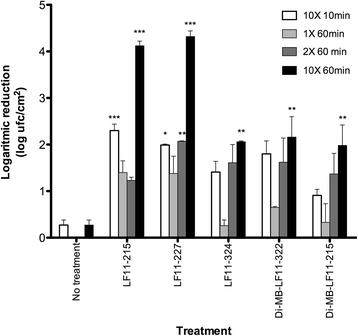
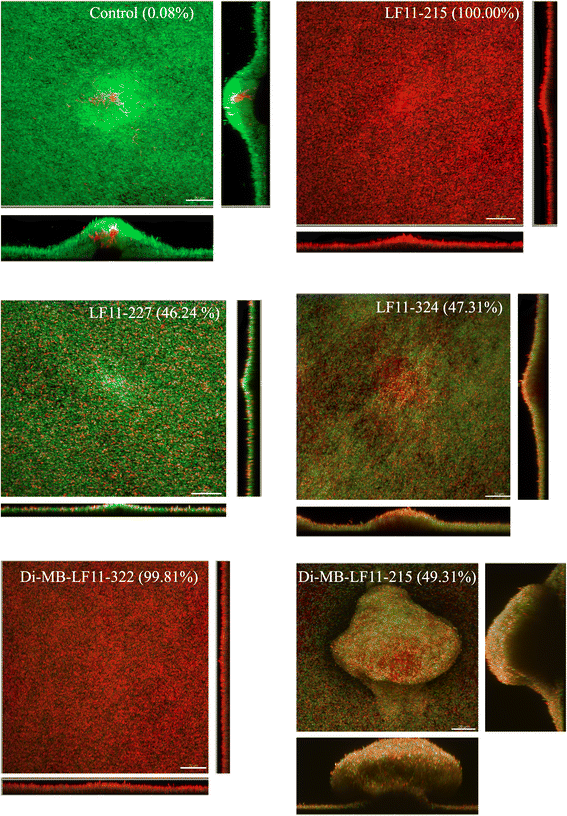
Results: The antimicrobial activity of lipopeptides differed from that of non-acylated peptides in their killing mechanisms on planktonic and biofilm-forming cells. Thus, acylation enhanced the bactericidal activity of the parental peptides and resulted in lipopeptides that were uniformly bactericidal at their MIC. In contrast, acylation of the most potent anti-biofilm peptides resulted in compounds with lower anti-biofilm activity. Both peptides and lipopeptides displayed very rapid killing kinetics and all of them required less than 21 min to reduce 1,000 times the viability of planktonic cells when tested at 2 times their MBC. The peptides, LF11-215 (FWRIRIRR) and LF11-227 (FWRRFWRR), displayed the most potent anti-biofilm activity causing a 10,000 fold reduction in cell viability after 1 h of treatment at 10 times their MIC. At that concentration, these two compounds exhibited low citotoxicity on human cells. In addition to its bactericidal activity, LF11-227 removed more that 50 % of the biofilm mass in independent assays. Peptide LF11-215 and two of the shortest and least hydrophobic lipopeptides, DI-MB-LF11-322 (2,2-dimethylbutanoyl-PFWRIRIRR) and DI-MB-LF11-215, penetrated deep into the biofilm structure and homogenously killed biofilm-forming bacteria.
Conclusion: We identified peptides derived from human lactoferricin with potent antimicrobial activity against P. aeruginosa growing either in planktonic or in biofilm mode. Although further structure-activity relationship analyses are necessary to optimize the anti-biofilm activity of these compounds, the results indicate that lactoferricin derived peptides are promising anti-biofilm agents.
Figures
Similar articles
-
Antibiofilm activity of lactoferrin-derived synthetic peptides against Pseudomonas aeruginosa PAO1.Biochem Cell Biol. 2021 Feb;99(1):138-148. doi: 10.1139/bcb-2020-0253. Epub 2020 Sep 1. Biochem Cell Biol. 2021. PMID: 32871093
-
Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides.Peptides. 2014 Dec;62:32-7. doi: 10.1016/j.peptides.2014.09.021. Epub 2014 Oct 5. Peptides. 2014. PMID: 25285879
-
Biofilm susceptibility to metal toxicity.Environ Microbiol. 2004 Dec;6(12):1220-7. doi: 10.1111/j.1462-2920.2004.00656.x. Environ Microbiol. 2004. PMID: 15560820
-
Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria.Biochim Biophys Acta. 2016 May;1858(5):1044-60. doi: 10.1016/j.bbamem.2015.10.013. Epub 2015 Oct 23. Biochim Biophys Acta. 2016. PMID: 26525663 Review.
-
Potential application of antimicrobial peptides in the treatment of bacterial biofilm infections.Curr Pharm Des. 2015;21(1):67-84. doi: 10.2174/1381612820666140905124312. Curr Pharm Des. 2015. PMID: 25189860 Review.
Cited by
-
Antibacterial Activity and Mechanism of Action of Bovine Lactoferricin Derivatives with Symmetrical Amino Acid Sequences.Int J Mol Sci. 2018 Sep 27;19(10):2951. doi: 10.3390/ijms19102951. Int J Mol Sci. 2018. PMID: 30262770 Free PMC article.
-
Antimicrobial Blue Light Inactivation of Microbial Isolates in Biofilms.Lasers Surg Med. 2020 Jun;52(5):472-478. doi: 10.1002/lsm.23159. Epub 2019 Sep 19. Lasers Surg Med. 2020. PMID: 31536154 Free PMC article.
-
Mammals' humoral immune proteins and peptides targeting the bacterial envelope: from natural protection to therapeutic applications against multidrug-resistant Gram-negatives.Biol Rev Camb Philos Soc. 2022 Jun;97(3):1005-1037. doi: 10.1111/brv.12830. Epub 2022 Jan 18. Biol Rev Camb Philos Soc. 2022. PMID: 35043558 Free PMC article. Review.
-
Lactoferrin in the Prevention and Treatment of Intestinal Inflammatory Pathologies Associated with Colorectal Cancer Development.Cancers (Basel). 2020 Dec 17;12(12):3806. doi: 10.3390/cancers12123806. Cancers (Basel). 2020. PMID: 33348646 Free PMC article. Review.
-
A synthetic peptide sensitizes multi-drug resistant Pseudomonas aeruginosa to antibiotics for more than two hours and permeabilizes its envelope for twenty hours.J Biomed Sci. 2020 Aug 6;27(1):85. doi: 10.1186/s12929-020-00678-3. J Biomed Sci. 2020. PMID: 32762680 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

