Physiologically normal 5% O2 supports neuronal differentiation and resistance to inflammatory injury in neural stem cell cultures
- PMID: 26147710
- PMCID: PMC4575628
- DOI: 10.1002/jnr.23615
Physiologically normal 5% O2 supports neuronal differentiation and resistance to inflammatory injury in neural stem cell cultures
Abstract
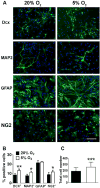
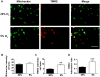
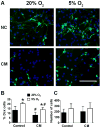
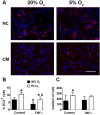
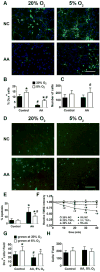
Recent studies have demonstrated that neural stem cell (NSC) culture at physiologically normoxic conditions (2-5% O2) is advantageous in terms of neuronal differentiation and survival. Neuronal differentiation is accompanied by a remarkable shift to mitochondrial oxidative metabolism compared with preferentially glycolytic metabolism of proliferating cells. However, metabolic changes induced by growth in a normoxic (5%) O2 culture environment in NSCs have been minimally explored. This study demonstrates that culturing under 5% O2 conditions results in higher levels of mitochondrial oxidative metabolism, decreased glycolysis, and reduced levels of reactive oxygen species in NSC cultures. Inflammation is one of the major environmental factors limiting postinjury NSC neuronal differentiation and survival. Our results show that NSCs differentiated under 5% O2 conditions possess better resistance to in vitro inflammatory injury compared with those exposed to 20% O2. The present work demonstrates that lower, more physiologically normal O2 levels support metabolic changes induced during NSC neuronal differentiation and provide increased resistance to inflammatory injury, thus highlighting O2 tension as an important determinant of cell fate and survival in various stem cell therapies.
Keywords: metabolism; mitochondria; neurogenesis; oxygen; stem cell.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
Figures

Similar articles
-
Adult neural stem cell fate is determined by thyroid hormone activation of mitochondrial metabolism.Mol Metab. 2017 Nov;6(11):1551-1561. doi: 10.1016/j.molmet.2017.08.003. Epub 2017 Aug 19. Mol Metab. 2017. PMID: 29107300 Free PMC article.
-
Functional Comparison of Neuronal Cells Differentiated from Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells under Different Oxygen and Medium Conditions.J Biomol Screen. 2016 Dec;21(10):1054-1064. doi: 10.1177/1087057116661291. Epub 2016 Aug 19. J Biomol Screen. 2016. PMID: 28139961
-
Differentiation-Dependent Energy Production and Metabolite Utilization: A Comparative Study on Neural Stem Cells, Neurons, and Astrocytes.Stem Cells Dev. 2016 Jul 1;25(13):995-1005. doi: 10.1089/scd.2015.0388. Epub 2016 Jun 7. Stem Cells Dev. 2016. PMID: 27116891 Free PMC article.
-
The role of oxygen in regulating neural stem cells in development and disease.J Cell Physiol. 2009 Sep;220(3):562-8. doi: 10.1002/jcp.21812. J Cell Physiol. 2009. PMID: 19441077 Review.
-
New Insights into the Role of Mild Hypoxia in Regulating Neural Stem Cell Characteristics.Stem Cells Dev. 2024 Jul;33(13-14):333-342. doi: 10.1089/scd.2024.0020. Epub 2024 Jun 11. Stem Cells Dev. 2024. PMID: 38753713 Review.
Cited by
-
Metabolic and Inflammatory Adaptation of Reactive Astrocytes: Role of PPARs.Mol Neurobiol. 2017 May;54(4):2518-2538. doi: 10.1007/s12035-016-9833-2. Epub 2016 Mar 17. Mol Neurobiol. 2017. PMID: 26984740 Review.
-
Organ-specific isogenic metastatic breast cancer cell lines exhibit distinct Raman spectral signatures and metabolomes.Oncotarget. 2017 Mar 21;8(12):20266-20287. doi: 10.18632/oncotarget.14865. Oncotarget. 2017. PMID: 28145887 Free PMC article.
-
Stem Cell-Derived Exosomes Protect Astrocyte Cultures From in vitro Ischemia and Decrease Injury as Post-stroke Intravenous Therapy.Front Cell Neurosci. 2019 Sep 3;13:394. doi: 10.3389/fncel.2019.00394. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31551712 Free PMC article.
-
Ferroptosis Contributes to Isoflurane Neurotoxicity.Front Mol Neurosci. 2019 Jan 9;11:486. doi: 10.3389/fnmol.2018.00486. eCollection 2018. Front Mol Neurosci. 2019. PMID: 30687003 Free PMC article.
-
Postinjury Inhibition of miR-181a Promotes Restoration of Hippocampal CA1 Neurons after Transient Forebrain Ischemia in Rats.eNeuro. 2019 Aug 29;6(4):ENEURO.0002-19.2019. doi: 10.1523/ENEURO.0002-19.2019. Print 2019 Jul/Aug. eNeuro. 2019. PMID: 31427401 Free PMC article.
References
-
- Borsini A, Zunszain PA, Thuret S, Pariante CM. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci 2015 - PubMed
-
- Brown GC, Borutaite V. Nitric oxide, mitochondria, and cell death. IUBMB Life. 2001;52(3-5):189–195. - PubMed
-
- Brundin P, Barker RA, Parmar M. Neural grafting in Parkinson's disease Problems and possibilities. Prog Brain Res. 2010;184:265–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

