STRATEGIC-1: A multiple-lines, randomized, open-label GERCOR phase III study in patients with unresectable wild-type RAS metastatic colorectal cancer
- PMID: 26141683
- PMCID: PMC4490616
- DOI: 10.1186/s12885-015-1503-7
STRATEGIC-1: A multiple-lines, randomized, open-label GERCOR phase III study in patients with unresectable wild-type RAS metastatic colorectal cancer
Abstract
Background: The management of unresectable metastatic colorectal cancer (mCRC) is a comprehensive treatment strategy involving several lines of therapy, maintenance, salvage surgery, and treatment-free intervals. Besides chemotherapy (fluoropyrimidine, oxaliplatin, irinotecan), molecular-targeted agents such as anti-angiogenic agents (bevacizumab, aflibercept, regorafenib) and anti-epidermal growth factor receptor agents (cetuximab, panitumumab) have become available. Ultimately, given the increasing cost of new active compounds, new strategy trials are needed to define the optimal use and the best sequencing of these agents. Such new clinical trials require alternative endpoints that can capture the effect of several treatment lines and be measured earlier than overall survival to help shorten the duration and reduce the size and cost of trials.
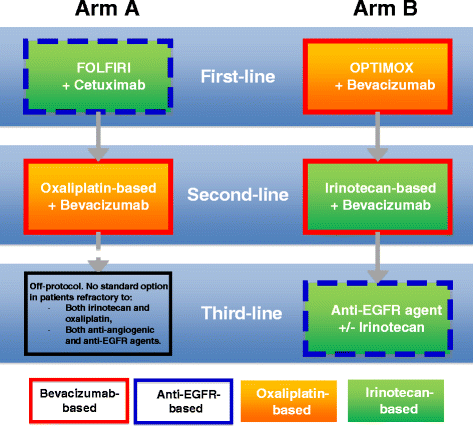
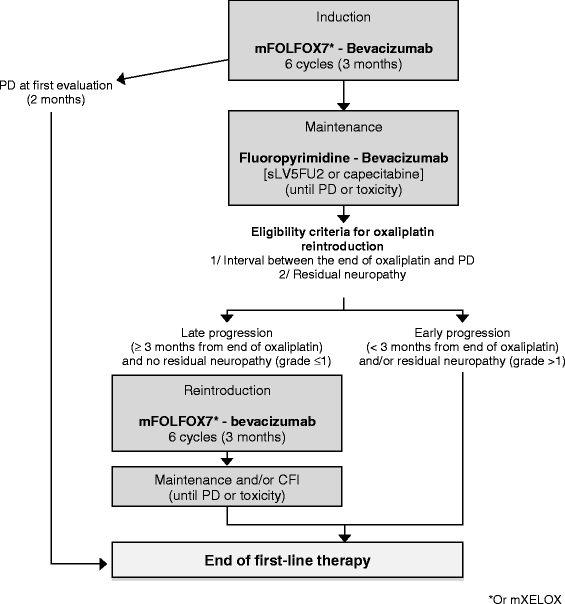
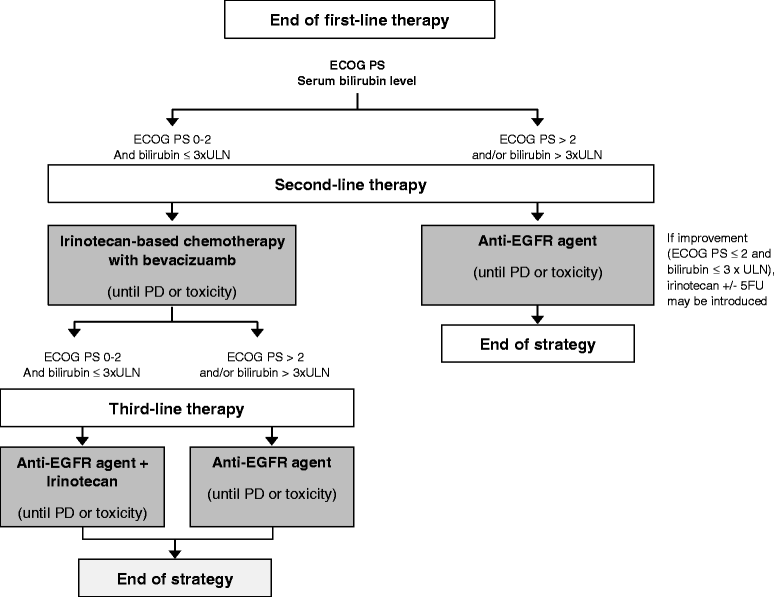
Methods/design: STRATEGIC-1 is an international, open-label, randomized, multicenter phase III trial designed to determine an optimally personalized treatment sequence of the available treatment modalities in patients with unresectable RAS wild-type mCRC. Two standard treatment strategies are compared: first-line FOLFIRI-cetuximab, followed by oxaliplatin-based second-line chemotherapy with bevacizumab (Arm A) vs. first-line OPTIMOX-bevacizumab, followed by irinotecan-based second-line chemotherapy with bevacizumab, and by an anti-epidermal growth factor receptor monoclonal antibody with or without irinotecan as third-line treatment (Arm B). The primary endpoint is duration of disease control. A total of 500 patients will be randomized in a 1:1 ratio to one of the two treatment strategies.
Discussion: The STRATEGIC-1 trial is designed to give global information on the therapeutic sequences in patients with unresectable RAS wild-type mCRC that in turn is likely to have a significant impact on the management of this patient population. The trial is open for inclusion since August 2013.
Trial registration: STRATEGIC-1 is registered at Clinicaltrials.gov: NCT01910610, 23 July, 2013. STRATEGIC-1 is registered at EudraCT-No.: 2013-001928-19, 25 April, 2013.
Figures
Similar articles
-
Continuation of Bevacizumab vs Cetuximab Plus Chemotherapy After First Progression in KRAS Wild-Type Metastatic Colorectal Cancer: The UNICANCER PRODIGE18 Randomized Clinical Trial.JAMA Oncol. 2019 Jan 1;5(1):83-90. doi: 10.1001/jamaoncol.2018.4465. JAMA Oncol. 2019. PMID: 30422156 Free PMC article. Clinical Trial.
-
SPIRITT: A Randomized, Multicenter, Phase II Study of Panitumumab with FOLFIRI and Bevacizumab with FOLFIRI as Second-Line Treatment in Patients with Unresectable Wild Type KRAS Metastatic Colorectal Cancer.Clin Colorectal Cancer. 2015 Jun;14(2):72-80. doi: 10.1016/j.clcc.2014.12.009. Epub 2015 Jan 8. Clin Colorectal Cancer. 2015. PMID: 25982297 Clinical Trial.
-
Activity and Safety of Cetuximab Plus Modified FOLFOXIRI Followed by Maintenance With Cetuximab or Bevacizumab for RAS and BRAF Wild-type Metastatic Colorectal Cancer: A Randomized Phase 2 Clinical Trial.JAMA Oncol. 2018 Apr 1;4(4):529-536. doi: 10.1001/jamaoncol.2017.5314. JAMA Oncol. 2018. PMID: 29450468 Free PMC article. Clinical Trial.
-
The clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal No.150 and part review of technology appraisal No. 118): a systematic review and economic model.Health Technol Assess. 2013 Apr;17(14):1-237. doi: 10.3310/hta17140. Health Technol Assess. 2013. PMID: 23547747 Free PMC article. Review.
-
Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review.Clin Ther. 2010 Mar;32(3):437-53. doi: 10.1016/j.clinthera.2010.03.012. Clin Ther. 2010. PMID: 20399983 Review.
Cited by
-
Treatment of Metastatic Colorectal Cancer: Standard of Care and Future Perspectives.Visc Med. 2016 Jun;32(3):178-83. doi: 10.1159/000446052. Epub 2016 Jun 7. Visc Med. 2016. PMID: 27493945 Free PMC article. Review.
-
Evaluation of Second-line Anti-VEGF after First-line Anti-EGFR Based Therapy in RAS Wild-Type Metastatic Colorectal Cancer: The Multicenter "SLAVE" Study.Cancers (Basel). 2020 May 16;12(5):1259. doi: 10.3390/cancers12051259. Cancers (Basel). 2020. PMID: 32429380 Free PMC article.
-
Sequential therapy with bevacizumab and EGFR inhibitors for metastatic colorectal carcinoma: a national registry-based analysis.Cancer Manag Res. 2018 Dec 28;11:359-368. doi: 10.2147/CMAR.S183093. eCollection 2019. Cancer Manag Res. 2018. PMID: 30643461 Free PMC article.
-
Immunotherapy for the treatment of colorectal tumors: focus on approved and in-clinical-trial monoclonal antibodies.Drug Des Devel Ther. 2017 Jan 11;11:177-184. doi: 10.2147/DDDT.S119036. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28138221 Free PMC article. Review.
-
Systematic Review and Meta-Analysis of Multitargeted Tyrosine Kinase Inhibitors in Patients With Intractable Metastatic Colorectal Cancer.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820943241. doi: 10.1177/1533033820943241. Technol Cancer Res Treat. 2020. PMID: 32914703 Free PMC article.
References
-
- de Gramont A, Bosset J, Milan C, Rougier P, Bouche O, Etienne P, Morvan F, Louvet C, Guillot T, Francois E, Bedenne L. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol. 1997;15(2):808–815. - PubMed
-
- Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, Bugat R, Findlay M, Frings S, Jahn M, McKendrick J, Osterwalder B, Perez-Manga G, Rosso R, Rougier P, Schmiegel WH, Seitz JF, Thompson P, Vieitez JM, Weitzel C, Harper P. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19(21):4097–4106. - PubMed
-
- de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–2947. - PubMed
-
- Goldberg R, Sargent D, Morton R, Fuchs C, Ramanathan R, Williamson S, Findlay B, Pitot H, Alberts S. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24(21):3347–3353. doi: 10.1200/JCO.2006.06.1317. - DOI - PubMed
-
- Diaz-Rubio E, Tabernero J, Gomez-Espana A, Massuti B, Sastre J, Chaves M, Abad A, Carrato A, Queralt B, Reina J, Maurel J, Gonzalez-Flores E, Aparicio J, Rivera F, Losa F, Aranda E. Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol. 2007;25(27):4224–4230. doi: 10.1200/JCO.2006.09.8467. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous