A Comparison of the Pathogenesis of Marburg Virus Disease in Humans and Nonhuman Primates and Evaluation of the Suitability of These Animal Models for Predicting Clinical Efficacy under the 'Animal Rule'
- PMID: 26141449
- PMCID: PMC4485633
A Comparison of the Pathogenesis of Marburg Virus Disease in Humans and Nonhuman Primates and Evaluation of the Suitability of These Animal Models for Predicting Clinical Efficacy under the 'Animal Rule'
Abstract
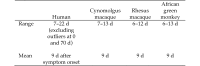
Marburg virus outbreaks are sporadic, infrequent, brief, and relatively small in terms of numbers of subjects affected. In addition, outbreaks most likely will occur in remote regions where clinical trials are not feasible; therefore, definitive, well-controlled human efficacy studies to test the effectiveness of a drug or biologic product are not feasible. Healthy human volunteers cannot ethically be deliberately exposed to a lethal agent such as Marburg virus in order to test the efficacy of a therapy or preventive prior to licensure. When human efficacy studies are neither ethical nor feasible, the US Food and Drug Administration may grant marketing approval of a drug or biologic product under the 'Animal Rule,' through which demonstration of the efficacy of a product can be 'based on adequate and well-controlled animal efficacy studies when the results of those studies establish that the drug is reasonably likely to produce clinical benefit in humans.' This process requires that the pathogenic determinants of the disease in the animal model are similar to those that have been identified in humans. After reviewing primarily English-language, peer-reviewed journal articles, we here summarize the clinical manifestations of Marburg virus disease and the results of studies in NHP showing the characteristics and progression of the disease. We also include a detailed comparison of the characteristics of the human disease relative to those for NHP. This review reveals that the disease characteristics of Marburg virus disease are generally similar for humans and 3 NHP species: cynomolgus macaques (Macaca fascicularis), rhesus macaques (Macaca mulatta), and African green monkeys (Chlorocebus aethiops).
Figures







Similar articles
-
Marburg virus pathogenesis - differences and similarities in humans and animal models.Virol J. 2019 Dec 30;16(1):165. doi: 10.1186/s12985-019-1272-z. Virol J. 2019. PMID: 31888676 Free PMC article. Review.
-
Transcriptional Profiling of the Immune Response to Marburg Virus Infection.J Virol. 2015 Oct;89(19):9865-74. doi: 10.1128/JVI.01142-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202234 Free PMC article.
-
Natural History of Aerosol Exposure with Marburg Virus in Rhesus Macaques.Viruses. 2016 Mar 30;8(4):87. doi: 10.3390/v8040087. Viruses. 2016. PMID: 27043611 Free PMC article.
-
Animal models for ebolavirus countermeasures discovery: what defines a useful model?Expert Opin Drug Discov. 2015 Jul;10(7):685-702. doi: 10.1517/17460441.2015.1035252. Epub 2015 May 25. Expert Opin Drug Discov. 2015. PMID: 26004783 Review.
-
AVI-7288 for Marburg Virus in Nonhuman Primates and Humans.N Engl J Med. 2015 Jul 23;373(4):339-48. doi: 10.1056/NEJMoa1410345. N Engl J Med. 2015. PMID: 26200980 Clinical Trial.
Cited by
-
New Insights Into Marburg Virus Disease Pathogenesis in the Rhesus Macaque Model.J Infect Dis. 2018 Nov 22;218(suppl_5):S423-S433. doi: 10.1093/infdis/jiy367. J Infect Dis. 2018. PMID: 30053050 Free PMC article.
-
Marburg and Ravn Virus Infections Do Not Cause Observable Disease in Ferrets.J Infect Dis. 2018 Nov 22;218(suppl_5):S471-S474. doi: 10.1093/infdis/jiy245. J Infect Dis. 2018. PMID: 29889278 Free PMC article.
-
Establishment and application of a surrogate model for human Ebola virus disease in BSL-2 laboratory.Virol Sin. 2024 Jun;39(3):434-446. doi: 10.1016/j.virs.2024.03.010. Epub 2024 Mar 29. Virol Sin. 2024. PMID: 38556051 Free PMC article.
-
A bioluminescent imaging mouse model for Marburg virus based on a pseudovirus system.Hum Vaccin Immunother. 2017 Aug 3;13(8):1811-1817. doi: 10.1080/21645515.2017.1325050. Epub 2017 May 8. Hum Vaccin Immunother. 2017. PMID: 28481728 Free PMC article.
-
Natural History of Marburg Virus Infection to Support Medical Countermeasure Development.Viruses. 2022 Oct 18;14(10):2291. doi: 10.3390/v14102291. Viruses. 2022. PMID: 36298846 Free PMC article.
References
-
- Adjemian J, Farnon EC, Tschioko F, Wamala JF, Byaruhanga E, Bwire GS, Kansiime E, Kagirita A, Ahimbisibwe S, Katunquka F, Jeff B, Lutwama JJ, Downing R, Tappero JW, Formenty P, Amman B, Manning C, Towner J, Nichol ST, Rollin PE. 2011. Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007. J Infect Dis 204:S796–S799. - PMC - PubMed
-
- Alves DA, Glynn AR, Steele KE, Lackemeyer MG, Garza NL, Buck JG, Mech C, Reed DS. 2010. Aerosol exposure to the Angola strain of Marburg virus causes lethal viral hemorrhagic fever in cynomolgus macaques. Vet Pathol 47:831–851. - PubMed
-
- Bausch DG, Nichol ST, Muyembe-Tamfum JJ, Borchert M, Rollin PE, Sleurs H, Campbell P, Tshioko FK, Roth C, Colebunders R, Pirard P, Mardel S, Olinda LA, Zeller H, Tshomba A, Kulidri A, Libande ML, Mulangu S, Formenty P, Grein T, Leirs H, Braack L, Ksiazek T, Zaki S, Bowen MD, Smit SB, Leman PA, Burt FJ, Kemp A, Swanepoel R. 2006. Marburg hemorrhagic fever associated with multiple genetic lineages of virus. N Engl J Med 355:909–919. - PubMed
-
- Bechtelsheimer H, Korb G, Gedigk P. 1972. The morphology and pathogenesis of ‘Marburg virus’ hepatitis. Hum Pathol 3:255–264. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
