CD8(+) T-cell Cytotoxic Capacity Associated with Human Immunodeficiency Virus-1 Control Can Be Mediated through Various Epitopes and Human Leukocyte Antigen Types
- PMID: 26137533
- PMCID: PMC4485486
- DOI: 10.1016/j.ebiom.2014.12.009
CD8(+) T-cell Cytotoxic Capacity Associated with Human Immunodeficiency Virus-1 Control Can Be Mediated through Various Epitopes and Human Leukocyte Antigen Types
Abstract
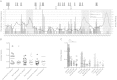
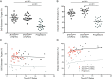
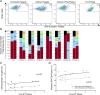
Understanding natural immunologic control over Human Immunodeficiency Virus (HIV)-1 replication, as occurs in rare long-term nonprogressors/elite controllers (LTNP/EC), should inform the design of efficacious HIV vaccines and immunotherapies. Durable control in LTNP/EC is likely mediated by highly functional virus-specific CD8(+) T-cells. Protective Human Leukocyte Antigen (HLA) class I alleles, like B*27 and B*57, are present in most, but not all LTNP/EC, providing an opportunity to investigate features shared by their HIV-specific immune responses. To better understand the contribution of epitope targeting and conservation to immune control, we compared the CD8(+) T-cell specificity and function of B*27/57(neg) LTNP/EC (n = 23), B*27/57(pos) LTNP/EC (n = 23) and B*27/57(neg) progressors (n = 13). Fine mapping revealed 11 previously unreported immunodominant responses. Although B*27/57(neg) LTNP/EC did not target more highly conserved epitopes, their CD8(+) T-cell cytotoxic capacity was significantly higher than progressors. Similar to B*27/57(pos) LTNP/EC, this superior cytotoxicity was mediated by preferential expansion of immunodominant responses and lysis through the predicted HLA. These findings suggest that increased CD8(+) T-cell cytotoxic capacity is a common mechanism of control in most LTNP/EC regardless of HLA type. They also suggest that potent cytotoxicity can be mediated through various epitopes and HLA molecules and could, in theory, be induced in most people.
Keywords: CD8+ T cells; Cytotoxic capacity; Epitope specificity; Immune control; Long-term nonprogressors/elite controllers.
Figures

Similar articles
-
The expansion ability but not the quality of HIV-specific CD8(+) T cells is associated with protective human leucocyte antigen class I alleles in long-term non-progressors.Immunology. 2011 Nov;134(3):305-13. doi: 10.1111/j.1365-2567.2011.03490.x. Immunology. 2011. PMID: 21978000 Free PMC article.
-
Efficacious early antiviral activity of HIV Gag- and Pol-specific HLA-B 2705-restricted CD8+ T cells.J Virol. 2010 Oct;84(20):10543-57. doi: 10.1128/JVI.00793-10. Epub 2010 Aug 4. J Virol. 2010. PMID: 20686036 Free PMC article.
-
HLA B*5701-positive long-term nonprogressors/elite controllers are not distinguished from progressors by the clonal composition of HIV-specific CD8+ T cells.J Virol. 2012 Apr;86(7):4014-8. doi: 10.1128/JVI.06982-11. Epub 2012 Jan 25. J Virol. 2012. PMID: 22278241 Free PMC article.
-
The influence of HLA/HIV genetics on the occurrence of elite controllers and a need for therapeutics geotargeting view.Braz J Infect Dis. 2021 Sep-Oct;25(5):101619. doi: 10.1016/j.bjid.2021.101619. Epub 2021 Sep 22. Braz J Infect Dis. 2021. PMID: 34562387 Free PMC article. Review.
-
The CD8+ T Cell Noncytotoxic Antiviral Responses.Microbiol Mol Biol Rev. 2021 May 12;85(2):e00155-20. doi: 10.1128/MMBR.00155-20. Print 2021 May 19. Microbiol Mol Biol Rev. 2021. PMID: 33980586 Free PMC article. Review.
Cited by
-
How elite controllers and posttreatment controllers inform our search for an HIV-1 cure.J Clin Invest. 2021 Jun 1;131(11):e149414. doi: 10.1172/JCI149414. J Clin Invest. 2021. PMID: 34060478 Free PMC article. Review.
-
Success and failure of the cellular immune response against HIV-1.Nat Immunol. 2015 Jun;16(6):563-70. doi: 10.1038/ni.3161. Nat Immunol. 2015. PMID: 25988888 Review.
-
A recombinant measles virus vaccine strongly reduces SHIV viremia and virus reservoir establishment in macaques.NPJ Vaccines. 2021 Oct 22;6(1):123. doi: 10.1038/s41541-021-00385-6. NPJ Vaccines. 2021. PMID: 34686669 Free PMC article.
-
Evaluation of antiviral T cell responses and TSCM cells in volunteers enrolled in a phase I HIV-1 subtype C prophylactic vaccine trial in India.PLoS One. 2020 Feb 25;15(2):e0229461. doi: 10.1371/journal.pone.0229461. eCollection 2020. PLoS One. 2020. PMID: 32097435 Free PMC article. Clinical Trial.
-
Metabolic pathway activation distinguishes transcriptional signatures of CD8+ T cells from HIV-1 elite controllers.AIDS. 2018 Nov 28;32(18):2669-2677. doi: 10.1097/QAD.0000000000002007. AIDS. 2018. PMID: 30289807 Free PMC article.
References
-
- Allen T.M., Altfeld M., Geer S.C., Kalife E.T., Moore C., O'Sullivan K.M., Desouza I., Feeney M.E., Eldridge R.L., Maier E.L. Selective escape from CD8 + T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J. Virol. 2005;79:13239–13249. - PMC - PubMed
-
- Altfeld M., Addo M.M., Rosenberg E.S., Hecht F.M., Lee P.K., Vogel M., Yu X.G., Draenert R., Johnston M.N., Strick D. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS. 2003;17:2581–2591. - PubMed
-
- Bansal A., Gough E., Sabbaj S., Ritter D., Yusim K., Sfakianos G., Aldrovandi G., Kaslow R.A., Wilson C.M., Mulligan M.J. CD8 T-cell responses in early HIV-1 infection are skewed towards high entropy peptides. AIDS. 2005;19:241–250. - PubMed
-
- Betts M.R., Ambrozak D.R., Douek D.C., Bonhoeffer S., Brenchley J.M., Casazza J.P., Koup R.A., Picker L.J. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 2001;75:11983–11991. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

