α6-Containing GABAA Receptors Are the Principal Mediators of Inhibitory Synapse Strengthening by Insulin in Cerebellar Granule Cells
- PMID: 26134650
- PMCID: PMC6605151
- DOI: 10.1523/JNEUROSCI.0513-15.2015
α6-Containing GABAA Receptors Are the Principal Mediators of Inhibitory Synapse Strengthening by Insulin in Cerebellar Granule Cells
Abstract
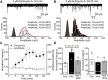
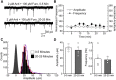
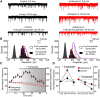
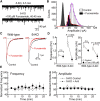
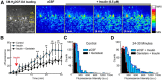
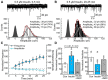
Activity-dependent strengthening of central synapses is a key factor driving neuronal circuit behavior in the vertebrate CNS. At fast inhibitory synapses, strengthening is thought to occur by increasing the number of GABAA receptors (GABARs) of the same subunit composition to preexisting synapses. Here, we show that strengthening of mouse cerebellar granule cell GABAergic synapses occurs by a different mechanism. Specifically, we show that the neuropeptide hormone, insulin, strengthens inhibitory synapses by recruiting α6-containing GABARs rather than accumulating more α1-containing receptors that are resident to the synapse. Because α6-receptors are targeted to functionally distinct postsynaptic sites from α1-receptors, we conclude that only a subset of all inhibitory synapses are strengthened. Together with our recent findings on stellate cells, we propose a general mechanism by which mature inhibitory synapses are strengthened. In this scenario, α1-GABARs resident to inhibitory synapses form the hardwiring of neuronal circuits with receptors of a different composition fulfilling a fundamental, but unappreciated, role in synapse strengthening.
Keywords: inhibition; insulin; metabolism; mitochondria; plasticity mechanism; reactive oxygen species.
Copyright © 2015 the authors 0270-6474/15/359676-13$15.00/0.
Figures


Similar articles
-
A reinforcing circuit action of extrasynaptic GABAA receptor modulators on cerebellar granule cell inhibition.PLoS One. 2013 Aug 19;8(8):e72976. doi: 10.1371/journal.pone.0072976. eCollection 2013. PLoS One. 2013. PMID: 23977374 Free PMC article.
-
Differential dependence of axo-dendritic and axo-somatic GABAergic synapses on GABAA receptors containing the alpha1 subunit in Purkinje cells.J Neurosci. 2006 Mar 22;26(12):3245-55. doi: 10.1523/JNEUROSCI.5118-05.2006. J Neurosci. 2006. PMID: 16554475 Free PMC article.
-
Neuroligin-2 accelerates GABAergic synapse maturation in cerebellar granule cells.Mol Cell Neurosci. 2009 Sep;42(1):45-55. doi: 10.1016/j.mcn.2009.05.004. Epub 2009 May 20. Mol Cell Neurosci. 2009. PMID: 19463950 Free PMC article.
-
α6-Containing GABAA Receptors: Functional Roles and Therapeutic Potentials.Pharmacol Rev. 2022 Jan;74(1):238-270. doi: 10.1124/pharmrev.121.000293. Pharmacol Rev. 2022. PMID: 35017178 Review.
-
Subunit composition of neurotransmitter receptors in the immature and in the epileptic brain.Biomed Res Int. 2014;2014:301950. doi: 10.1155/2014/301950. Epub 2014 Sep 11. Biomed Res Int. 2014. PMID: 25295256 Free PMC article. Review.
Cited by
-
Exploiting the Indole Scaffold to Design Compounds Binding to Different Pharmacological Targets.Molecules. 2020 May 16;25(10):2331. doi: 10.3390/molecules25102331. Molecules. 2020. PMID: 32429433 Free PMC article. Review.
-
Control of motor coordination by astrocytic tonic GABA release through modulation of excitation/inhibition balance in cerebellum.Proc Natl Acad Sci U S A. 2018 May 8;115(19):5004-5009. doi: 10.1073/pnas.1721187115. Epub 2018 Apr 24. Proc Natl Acad Sci U S A. 2018. PMID: 29691318 Free PMC article.
-
Gene-body 5-hydroxymethylation is associated with gene expression changes in the prefrontal cortex of depressed individuals.Transl Psychiatry. 2017 May 9;7(5):e1119. doi: 10.1038/tp.2017.93. Transl Psychiatry. 2017. PMID: 28485726 Free PMC article.
-
Two Distinct Populations of α1α6-Containing GABAA-Receptors in Rat Cerebellum.Front Synaptic Neurosci. 2020 Oct 6;12:591129. doi: 10.3389/fnsyn.2020.591129. eCollection 2020. Front Synaptic Neurosci. 2020. PMID: 33123001 Free PMC article.
-
Assembly rules for GABAA receptor complexes in the brain.Elife. 2017 Aug 17;6:e27443. doi: 10.7554/eLife.27443. Elife. 2017. PMID: 28816653 Free PMC article.
References
-
- Barnard EA, Skolnick P, Olsen RW, Möhler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology: XV. Subtypes of gamma-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
