Aldose Reductase Mediates Transforming Growth Factor β2 (TGF-β2)-Induced Migration and Epithelial-To-Mesenchymal Transition of Lens-Derived Epithelial Cells
- PMID: 26132779
- PMCID: PMC4495811
- DOI: 10.1167/iovs.15-16557
Aldose Reductase Mediates Transforming Growth Factor β2 (TGF-β2)-Induced Migration and Epithelial-To-Mesenchymal Transition of Lens-Derived Epithelial Cells
Abstract
Purpose: Cataract surgery involves removal of lens tissue, but is associated with a high complication rate due to regrowth of residual lens epithelial cells to produce posterior capsule opacification (PCO) and diminished visual acuity. As inhibitors of aldose reductase (AR) have been shown to suppress markers of PCO, our studies were designed to identify a role for AR in the pathogenesis of PCO.
Methods: Sorbinil-mediated AR inhibition was determined by measuring sorbitol accumulation. Cell migration was measured using both transwell and scratch assays. Proteins in the SMAD signaling pathway were measured by Western blotting. The interactions of AR and SMADs were demonstrated by co-immunoprecipitation (Co-IP) and proximity ligation assay (PLA). Epithelial-to-mesenchymal transition (EMT) expression was measured by Western blot and quantitative PCR (q-PCR). Matrix metalloproteinase (MMP)-2 and MMP-9 activities were measured in conditioned medium by zymography.
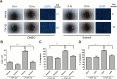
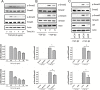
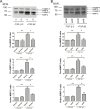
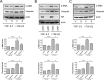
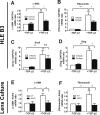
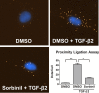
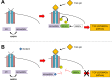
Results: We observed that either Sorbinil-mediated AR inhibition or siRNA-mediated AR gene knockdown prevented migration of lens epithelial cells following exposure to TGF-β2. AR inhibition or AR knockdown reduced SMAD and MMP activation triggered by TGF-β2. In addition, we demonstrated AR inhibition or AR knockdown decreased TGF-β2-induced expression of EMT markers. Co-IP studies and PLA were used to demonstrate that AR and SMAD2 interact either directly or in close concert with additional factor(s) in a nonenzymatic manner.
Conclusions: This study demonstrates that AR participates in the response of lens epithelial cells to TGF-β2. Our studies raise the possibility that AR inhibition may be effective in preventing development of PCO by disrupting the TGF-β2/SMAD pathway.
Figures

Similar articles
-
Implication of Smad2 and Smad3 in transforming growth factor-β-induced posterior capsular opacification of human lens epithelial cells.Curr Eye Res. 2015 Apr;40(4):386-97. doi: 10.3109/02713683.2014.925932. Epub 2014 Jun 9. Curr Eye Res. 2015. PMID: 24911914
-
Influence of aldose reductase on epithelial-to-mesenchymal transition signaling in lens epithelial cells.Chem Biol Interact. 2017 Oct 1;276:149-154. doi: 10.1016/j.cbi.2017.01.017. Epub 2017 Jan 27. Chem Biol Interact. 2017. PMID: 28137510 Free PMC article.
-
Bit1-a potential positive regulator of epithelial-mesenchymal transition in lens epithelial cells.Graefes Arch Clin Exp Ophthalmol. 2016 Jul;254(7):1311-8. doi: 10.1007/s00417-016-3357-3. Epub 2016 Apr 28. Graefes Arch Clin Exp Ophthalmol. 2016. PMID: 27122244
-
Roles of TGF β and FGF Signals in the Lens: Tropomyosin Regulation for Posterior Capsule Opacity.Int J Mol Sci. 2018 Oct 9;19(10):3093. doi: 10.3390/ijms19103093. Int J Mol Sci. 2018. PMID: 30304871 Free PMC article. Review.
-
Fibrosis in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT leading to cataract.Exp Eye Res. 2016 Jan;142:92-101. doi: 10.1016/j.exer.2015.02.004. Epub 2015 May 21. Exp Eye Res. 2016. PMID: 26003864 Free PMC article. Review.
Cited by
-
Diabetes-Independent Retinal Phenotypes in an Aldose Reductase Transgenic Mouse Model.Metabolites. 2021 Jul 10;11(7):450. doi: 10.3390/metabo11070450. Metabolites. 2021. PMID: 34357344 Free PMC article.
-
The Role of Metalloproteinases and Their Tissue Inhibitors on Ocular Diseases: Focusing on Potential Mechanisms.Int J Mol Sci. 2022 Apr 12;23(8):4256. doi: 10.3390/ijms23084256. Int J Mol Sci. 2022. PMID: 35457074 Free PMC article. Review.
-
Aldose Reductase Inhibition Prevents Development of Posterior Capsular Opacification in an In Vivo Model of Cataract Surgery.Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3591-3598. doi: 10.1167/iovs.18-23935. Invest Ophthalmol Vis Sci. 2018. PMID: 30025084 Free PMC article.
-
miR-30a reverses TGF-β2-induced migration and EMT in posterior capsular opacification by targeting Smad2.Mol Biol Rep. 2019 Aug;46(4):3899-3907. doi: 10.1007/s11033-019-04833-4. Epub 2019 May 2. Mol Biol Rep. 2019. PMID: 31049834
-
αB-crystallin is essential for the TGF-β2-mediated epithelial to mesenchymal transition of lens epithelial cells.Biochem J. 2016 May 15;473(10):1455-69. doi: 10.1042/BCJ20160128. Epub 2016 Mar 17. Biochem J. 2016. PMID: 26987815 Free PMC article.
References
-
- Dewey S. Posterior capsule opacification. Curr Opin Ophthalmol. 2006; 17: 45–53. - PubMed
-
- de Iongh RU,, Wederell E,, Lovicu FJ,, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005; 179: 43–55. - PubMed
-
- Wormstone IM. Posterior capsule opacification: a cell biological perspective. Exp Eye Res. 2002; 74: 337–347. - PubMed
-
- Wormstone IM,, Tamiya S,, Anderson I,, Duncan G. TGF-beta2–induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci. 2002; 43: 2301–2308. - PubMed
-
- Gotoh N,, Perdue NR,, Matsushima H,, Sage EH,, Yan Q,, Clark JI. An in vitro model of posterior capsular opacity: SPARC and TGF-beta2 minimize epithelial-to-mesenchymal transition in lens epithelium. Invest Ophthalmol Vis Sci. 2007; 48: 4679–4687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

