Study of Protein Phosphatase 2A (PP2A) Activity in LPS-Induced Tolerance Using Fluorescence-Based and Immunoprecipitation-Aided Methodology
- PMID: 26131975
- PMCID: PMC4598752
- DOI: 10.3390/biom5031284
Study of Protein Phosphatase 2A (PP2A) Activity in LPS-Induced Tolerance Using Fluorescence-Based and Immunoprecipitation-Aided Methodology
Abstract
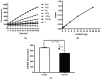
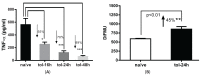
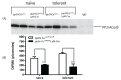
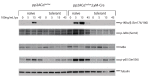
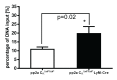
Protein phosphatase 2A (PP2A) is one of the most abundant intracellular serine/threonine (Ser/Thr) phosphatases accounting for 1% of the total cellular protein content. PP2A is comprised of a heterodimeric core enzyme and a substrate-specific regulatory subunit. Potentially, at least seventy different compositions of PP2A exist because of variable regulatory subunit binding that accounts for various activity modulating numerous cell functions. Due to the constitutive phosphatase activity present inside cells, a sensitive assay is required to detect the changes of PP2A activity under various experimental conditions. We optimized a fluorescence assay (DIFMU assay) by combining it with prior anti-PP2A immunoprecipitation to quantify PP2A-specific phosphatase activity. It is also known that prior exposure to lipopolysaccharides (LPS) induces "immune tolerance" of the cells to subsequent stimulation. Herein we report that PP2A activity is upregulated in tolerized peritoneal macrophages, corresponding to decreased TNF-α secretion upon second LPS stimulation. We further examined the role of PP2A in the tolerance effect by using PP2ACαl°xl°x;lyM-Cre conditional knockout macrophages. We found that PP2A phosphatase activity cannot be further increased by tolerance. TNF-α secretion from tolerized PP2ACαl°xl°x;lyM-Cre macrophages is higher than tolerized control macrophages. Furthermore, we showed that the increased TNF-α secretion may be due to an epigenetic transcriptionally active signature on the promoter of TNF-α gene rather than regulation of the NFκB/IκB signaling pathway. These results suggest a role for increased PP2A activity in the regulation of immune tolerance.
Keywords: PP2A; immune tolerance; phosphatase assay.
Figures




Similar articles
-
Myeloid-Specific Gene Deletion of Protein Phosphatase 2A Magnifies MyD88- and TRIF-Dependent Inflammation following Endotoxin Challenge.J Immunol. 2017 Jan 1;198(1):404-416. doi: 10.4049/jimmunol.1600221. Epub 2016 Nov 21. J Immunol. 2017. PMID: 27872207 Free PMC article.
-
[KAT6B promotes LPS-triggered IL-6 production via enhancing recruitment of H3K23 acetylation to IL-6 promoter region].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017 Nov;33(11):1441-147. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017. PMID: 29268844 Chinese.
-
Okadaic acid stimulates inflammatory cytokine gene transcription in murine peritoneal macrophages.Cell Immunol. 1994 Feb;153(2):479-91. doi: 10.1006/cimm.1994.1044. Cell Immunol. 1994. PMID: 8118877
-
Physiologic functions of PP2A: Lessons from genetically modified mice.Biochim Biophys Acta Mol Cell Res. 2019 Jan;1866(1):31-50. doi: 10.1016/j.bbamcr.2018.07.010. Epub 2018 Jul 18. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30030003 Review.
-
Protein Phosphatase 2A: a Double-Faced Phosphatase of Cellular System and Its Role in Neurodegenerative Disorders.Mol Neurobiol. 2018 Feb;55(2):1750-1761. doi: 10.1007/s12035-017-0444-3. Epub 2017 Feb 21. Mol Neurobiol. 2018. PMID: 28224476 Review.
Cited by
-
CBP Bromodomain Inhibition Rescues Mice From Lethal Sepsis Through Blocking HMGB1-Mediated Inflammatory Responses.Front Immunol. 2021 Feb 2;11:625542. doi: 10.3389/fimmu.2020.625542. eCollection 2020. Front Immunol. 2021. PMID: 33603756 Free PMC article.
-
PPP2R5D promotes hepatitis C virus infection by binding to viral NS5B and enhancing viral RNA replication.Virol J. 2022 Jul 14;19(1):118. doi: 10.1186/s12985-022-01848-5. Virol J. 2022. PMID: 35836293 Free PMC article.
-
Could Alzheimer's Disease Originate in the Periphery and If So How So?Mol Neurobiol. 2019 Jan;56(1):406-434. doi: 10.1007/s12035-018-1092-y. Epub 2018 Apr 29. Mol Neurobiol. 2019. PMID: 29705945 Free PMC article. Review.
-
Role of CD44 in Regulating TLR2 Activation of Human Macrophages and Downstream Expression of Proinflammatory Cytokines.J Immunol. 2018 Jan 15;200(2):758-767. doi: 10.4049/jimmunol.1700713. Epub 2017 Dec 1. J Immunol. 2018. PMID: 29196459 Free PMC article.
-
CD44 Receptor Mediates Urate Crystal Phagocytosis by Macrophages and Regulates Inflammation in A Murine Peritoneal Model of Acute Gout.Sci Rep. 2020 Apr 1;10(1):5748. doi: 10.1038/s41598-020-62727-z. Sci Rep. 2020. PMID: 32238827 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

