Selectivity is species-dependent: Characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors
- PMID: 26126429
- PMCID: PMC4529847
- DOI: 10.1007/s11302-015-9460-9
Selectivity is species-dependent: Characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors
Abstract
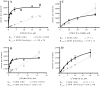
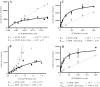
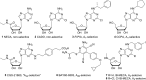
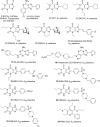
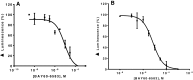
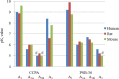
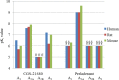
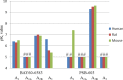
Adenosine receptors (ARs) have emerged as new drug targets. The majority of data on affinity/potency and selectivity of AR ligands described in the literature has been obtained for the human species. However, preclinical studies are mostly performed in mouse or rat, and standard AR agonists and antagonists are frequently used for studies in rodents without knowing their selectivity in the investigated species. In the present study, we selected a set of frequently used standard AR ligands, 8 agonists and 16 antagonists, and investigated them in radioligand binding studies at all four AR subtypes, A1, A2A, A2B, and A3, of three species, human, rat, and mouse. Recommended, selective agonists include CCPA (for A1AR of rat and mouse), CGS-21680 (for A2A AR of rat), and Cl-IB-MECA (for A3AR of all three species). The functionally selective partial A2B agonist BAY60-6583 was found to additionally bind to A1 and A3AR and act as an antagonist at both receptor subtypes. The antagonists PSB-36 (A1), preladenant (A2A), and PSB-603 (A2B) displayed high selectivity in all three investigated species. MRS-1523 acts as a selective A3AR antagonist in human and rat, but is only moderately selective in mouse. The comprehensive data presented herein provide a solid basis for selecting suitable AR ligands for biological studies.
Figures

Similar articles
-
Targeting adenosine receptors with coumarins: synthesis and binding activities of amide and carbamate derivatives.J Pharm Pharmacol. 2013 Jan;65(1):30-4. doi: 10.1111/j.2042-7158.2012.01571.x. J Pharm Pharmacol. 2013. PMID: 23215685
-
Stimulation of the adenosine A3 receptor, not the A1 or A2 receptors, promote neurite outgrowth of retinal ganglion cells.Exp Eye Res. 2018 May;170:160-168. doi: 10.1016/j.exer.2018.02.019. Epub 2018 Feb 24. Exp Eye Res. 2018. PMID: 29486164
-
2-Substituted adenosine derivatives: affinity and efficacy at four subtypes of human adenosine receptors.Biochem Pharmacol. 2004 Nov 15;68(10):1985-93. doi: 10.1016/j.bcp.2004.06.011. Biochem Pharmacol. 2004. PMID: 15476669 Free PMC article.
-
Blood Platelet Adenosine Receptors as Potential Targets for Anti-Platelet Therapy.Int J Mol Sci. 2019 Nov 3;20(21):5475. doi: 10.3390/ijms20215475. Int J Mol Sci. 2019. PMID: 31684173 Free PMC article. Review.
-
Adenosine and adenosine receptors in the immunopathogenesis and treatment of cancer.J Cell Physiol. 2018 Mar;233(3):2032-2057. doi: 10.1002/jcp.25873. Epub 2017 May 3. J Cell Physiol. 2018. PMID: 28233320 Review.
Cited by
-
New paradigms in purinergic receptor ligand discovery.Neuropharmacology. 2023 Jun 1;230:109503. doi: 10.1016/j.neuropharm.2023.109503. Epub 2023 Mar 13. Neuropharmacology. 2023. PMID: 36921890 Free PMC article. Review.
-
Irreversible Antagonists for the Adenosine A2B Receptor.Molecules. 2022 Jun 13;27(12):3792. doi: 10.3390/molecules27123792. Molecules. 2022. PMID: 35744918 Free PMC article.
-
Pharmacological characterization of DPTN and other selective A3 adenosine receptor antagonists.Purinergic Signal. 2021 Dec;17(4):737-746. doi: 10.1007/s11302-021-09823-5. Epub 2021 Oct 28. Purinergic Signal. 2021. PMID: 34713378 Free PMC article.
-
Adenosine Receptor-Mediated Cardioprotection-Current Limitations and Future Directions.Front Pharmacol. 2018 Apr 4;9:310. doi: 10.3389/fphar.2018.00310. eCollection 2018. Front Pharmacol. 2018. PMID: 29670529 Free PMC article.
-
Optical Control of Adenosine-Mediated Pain Modulation.Bioconjug Chem. 2021 Sep 15;32(9):1979-1983. doi: 10.1021/acs.bioconjchem.1c00387. Epub 2021 Aug 27. Bioconjug Chem. 2021. PMID: 34448572 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

