Neural activity and CaMKII protect mitochondria from fragmentation in aging Caenorhabditis elegans neurons
- PMID: 26124107
- PMCID: PMC4507213
- DOI: 10.1073/pnas.1501831112
Neural activity and CaMKII protect mitochondria from fragmentation in aging Caenorhabditis elegans neurons
Abstract
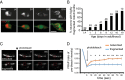
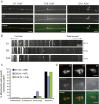
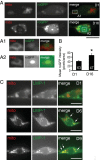
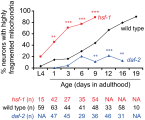
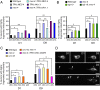
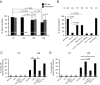
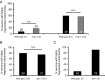
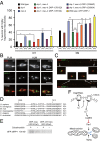
Decline in mitochondrial morphology and function is a hallmark of neuronal aging. Here we report that progressive mitochondrial fragmentation is a common manifestation of aging Caenorhabditis elegans neurons and body wall muscles. We show that sensory-evoked activity was essential for maintaining neuronal mitochondrial morphology, and this activity-dependent mechanism required the Degenerin/ENaC sodium channel MEC-4, the L-type voltage-gated calcium channel EGL-19, and the Ca/calmodulin-dependent kinase II (CaMKII) UNC-43. Importantly, UNC-43 phosphorylated and inhibited the dynamin-related protein (DRP)-1, which was responsible for excessive mitochondrial fragmentation in neurons that lacked sensory-evoked activity. Moreover, enhanced activity in the aged neurons ameliorated mitochondrial fragmentation. These findings provide a detailed description of mitochondrial behavior in aging neurons and identify activity-dependent DRP-1 phosphorylation by CaMKII as a key mechanism in neuronal mitochondrial maintenance.
Keywords: C. elegans; CaMKII; mitochondria; neural activity; neuronal aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

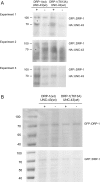
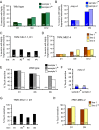
Similar articles
-
The Caenorhabditis elegans voltage-gated calcium channel subunits UNC-2 and UNC-36 and the calcium-dependent kinase UNC-43/CaMKII regulate neuromuscular junction morphology.Neural Dev. 2013 May 10;8:10. doi: 10.1186/1749-8104-8-10. Neural Dev. 2013. PMID: 23663262 Free PMC article.
-
Neuronal Activity and CaMKII Regulate Kinesin-Mediated Transport of Synaptic AMPARs.Neuron. 2015 Apr 22;86(2):457-74. doi: 10.1016/j.neuron.2015.03.011. Epub 2015 Apr 2. Neuron. 2015. PMID: 25843407 Free PMC article.
-
Mitochondrial stress extends lifespan in C. elegans through neuronal hormesis.Exp Gerontol. 2014 Aug;56:89-98. doi: 10.1016/j.exger.2014.03.026. Epub 2014 Apr 4. Exp Gerontol. 2014. PMID: 24709340
-
Mitochondrial influence on aging rate in Caenorhabditis elegans.Aging Cell. 2004 Feb;3(1):29-34. doi: 10.1111/j.1474-9728.2003.00077.x. Aging Cell. 2004. PMID: 14965353 Review.
-
Collaboration between mitochondria and the nucleus is key to long life in Caenorhabditis elegans.Free Radic Biol Med. 2015 Jan;78:168-78. doi: 10.1016/j.freeradbiomed.2014.10.576. Epub 2014 Nov 4. Free Radic Biol Med. 2015. PMID: 25450327 Free PMC article. Review.
Cited by
-
Age-dependent changes in response property and morphology of a thermosensory neuron and thermotaxis behavior in Caenorhabditis elegans.Aging Cell. 2020 May;19(5):e13146. doi: 10.1111/acel.13146. Epub 2020 Apr 19. Aging Cell. 2020. PMID: 32307902 Free PMC article.
-
The Energy Maintenance Theory of Aging: Maintaining Energy Metabolism to Allow Longevity.Bioessays. 2018 Aug;40(8):e1800005. doi: 10.1002/bies.201800005. Epub 2018 Jun 14. Bioessays. 2018. PMID: 29901833 Free PMC article. Review.
-
BAM15 Relieves Neurodegeneration in Aged Caenorhabditis elegans and Extends Lifespan.Metabolites. 2022 Nov 17;12(11):1129. doi: 10.3390/metabo12111129. Metabolites. 2022. PMID: 36422268 Free PMC article.
-
In Vivo Imaging with Genetically Encoded Redox Biosensors.Int J Mol Sci. 2020 Oct 31;21(21):8164. doi: 10.3390/ijms21218164. Int J Mol Sci. 2020. PMID: 33142884 Free PMC article. Review.
-
Tauopathy-associated tau modifications selectively impact neurodegeneration and mitophagy in a novel C. elegans single-copy transgenic model.Mol Neurodegener. 2020 Nov 9;15(1):65. doi: 10.1186/s13024-020-00410-7. Mol Neurodegener. 2020. PMID: 33168053 Free PMC article.
References
-
- Chan DC. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. - PubMed
-
- Scheckhuber CQ, et al. Reducing mitochondrial fission results in increased life span and fitness of two fungal ageing models. Nat Cell Biol. 2007;9(1):99–105. - PubMed
-
- Wilson PD, Franks LM. The effect of age on mitochondrial ultrastructure. Gerontologia. 1975;21(2):81–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

