Role of Regulators of G Protein Signaling Proteins in Bone Physiology and Pathophysiology
- PMID: 26123302
- PMCID: PMC4817727
- DOI: 10.1016/bs.pmbts.2015.02.002
Role of Regulators of G Protein Signaling Proteins in Bone Physiology and Pathophysiology
Abstract
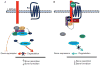
Regulators of G protein signaling (RGS) proteins enhance the intrinsic GTPase activity of α subunits of the heterotrimeric G protein complex of G protein-coupled receptors (GPCRs) and thereby inactivate signal transduction initiated by GPCRs. The RGS family consists of nearly 37 members with a conserved RGS homology domain which is critical for their GTPase accelerating activity. RGS proteins are expressed in most tissues, including heart, lung, brain, kidney, and bone and play essential roles in many physiological and pathological processes. In skeletal development and bone homeostasis as well as in many bone disorders, RGS proteins control the functions of various GPCRs, including the parathyroid hormone receptor type 1 and calcium-sensing receptor and also regulate various critical signaling pathways, such as Wnt and calcium oscillations. This chapter will discuss the current findings on the roles of RGS proteins in regulating signaling of key GPCRs in skeletal development and bone homeostasis. We also will examine the current updates of RGS proteins' regulation of calcium oscillations in bone physiology and highlight the roles of RGS proteins in selected bone pathological disorders. Despite the recent advances in bone and mineral research, RGS proteins remain understudied in the skeletal system. Further understanding of the roles of RGS proteins in bone should not only provide great insights into the molecular basis of various bone diseases but also generate great therapeutic drug targets for many bone diseases.
Keywords: Bone; Bone homeostasis; G protein-coupled receptors; G proteins; Osteoblasts; Osteoclasts; PTH/PTHrP; Regulators of G protein signaling; Skeletal disorders; Wnt.
© 2015 Elsevier Inc. All rights reserved.
Figures
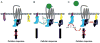

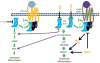
Similar articles
-
Introduction: G Protein-coupled Receptors and RGS Proteins.Prog Mol Biol Transl Sci. 2015;133:1-11. doi: 10.1016/bs.pmbts.2015.03.002. Epub 2015 Apr 8. Prog Mol Biol Transl Sci. 2015. PMID: 26123299 Review.
-
Role of regulator of G protein signaling proteins in bone.Front Biosci (Landmark Ed). 2014 Jan 1;19(4):634-48. doi: 10.2741/4232. Front Biosci (Landmark Ed). 2014. PMID: 24389209 Free PMC article. Review.
-
Effect of Regulator of G Protein Signaling Proteins on Bone.Front Endocrinol (Lausanne). 2022 Apr 29;13:842421. doi: 10.3389/fendo.2022.842421. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35573989 Free PMC article. Review.
-
Regulators of G-protein-signaling proteins: negative modulators of G-protein-coupled receptor signaling.Int Rev Cell Mol Biol. 2015;317:97-183. doi: 10.1016/bs.ircmb.2015.02.001. Epub 2015 Mar 5. Int Rev Cell Mol Biol. 2015. PMID: 26008785 Review.
-
Vitamin D and dexamethasone inversely regulate parathyroid hormone-induced regulator of G protein signaling-2 expression in osteoblast-like cells.Endocrinology. 2003 Jun;144(6):2496-504. doi: 10.1210/en.2002-0160. Endocrinology. 2003. PMID: 12746312
Cited by
-
Identification and experimental validation of hub genes underlying depressive-like behaviors induced by chronic social defeat stress.Front Pharmacol. 2024 Oct 14;15:1472468. doi: 10.3389/fphar.2024.1472468. eCollection 2024. Front Pharmacol. 2024. PMID: 39469623 Free PMC article.
-
Metabolic acidosis regulates RGS16 and G protein signaling in osteoblasts.Am J Physiol Renal Physiol. 2021 Oct 1;321(4):F424-F430. doi: 10.1152/ajprenal.00166.2021. Epub 2021 Aug 16. Am J Physiol Renal Physiol. 2021. PMID: 34396788 Free PMC article.
-
Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro.Tissue Eng Part B Rev. 2017 Jun;23(3):268-280. doi: 10.1089/ten.TEB.2016.0454. Epub 2016 Dec 27. Tissue Eng Part B Rev. 2017. PMID: 27846781 Free PMC article.
-
Hedgehog Signaling Inhibition by Smoothened Antagonist BMS-833923 Reduces Osteoblast Differentiation and Ectopic Bone Formation of Human Skeletal (Mesenchymal) Stem Cells.Stem Cells Int. 2019 Nov 21;2019:3435901. doi: 10.1155/2019/3435901. eCollection 2019. Stem Cells Int. 2019. PMID: 31871467 Free PMC article.
-
Expression regulation and functional analysis of RGS2 and RGS4 in adipogenic and osteogenic differentiation of human mesenchymal stem cells.Biol Res. 2017 Dec 26;50(1):43. doi: 10.1186/s40659-017-0148-1. Biol Res. 2017. PMID: 29279050 Free PMC article.
References
-
- Boyce BF, Rosenberg E, de Papp AE, Duong le T. The osteoclast, bone remodelling and treatment of metabolic bone disease. Eur J Clin Invest. 2012;42(12):1332–1341. - PubMed
-
- Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40(2):251–264. - PubMed
-
- Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

