IL-34 mediates acute kidney injury and worsens subsequent chronic kidney disease
- PMID: 26121749
- PMCID: PMC4563757
- DOI: 10.1172/JCI81166
IL-34 mediates acute kidney injury and worsens subsequent chronic kidney disease
Abstract
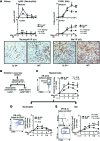
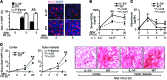
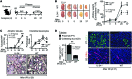
Macrophages (Mø) are integral in ischemia/reperfusion injury-incited (I/R-incited) acute kidney injury (AKI) that leads to fibrosis and chronic kidney disease (CKD). IL-34 and CSF-1 share a receptor (c-FMS), and both cytokines mediate Mø survival and proliferation but also have distinct features. CSF-1 is central to kidney repair and destruction. We tested the hypothesis that IL-34-dependent, Mø-mediated mechanisms promote persistent ischemia-incited AKI that worsens subsequent CKD. In renal I/R, the time-related magnitude of Mø-mediated AKI and subsequent CKD were markedly reduced in IL-34-deficient mice compared with controls. IL-34, c-FMS, and a second IL-34 receptor, protein-tyrosine phosphatase ζ (PTP-ζ) were upregulated in the kidney after I/R. IL-34 was generated by tubular epithelial cells (TECs) and promoted Mø-mediated TEC destruction during AKI that worsened subsequent CKD via 2 distinct mechanisms: enhanced intrarenal Mø proliferation and elevated BM myeloid cell proliferation, which increases circulating monocytes that are drawn into the kidney by chemokines. CSF-1 expression in TECs did not compensate for IL-34 deficiency. In patients, kidney transplants subject to I/R expressed IL-34, c-FMS, and PTP-ζ in TECs during AKI that increased with advancing injury. Moreover, IL-34 expression increased, along with more enduring ischemia in donor kidneys. In conclusion, IL-34-dependent, Mø-mediated, CSF-1 nonredundant mechanisms promote persistent ischemia-incited AKI that worsens subsequent CKD.
Figures
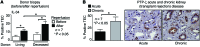








Comment in
-
Acute kidney injury: IL-34 promotes persistent ischaemia-induced AKI.Nat Rev Nephrol. 2015 Sep;11(9):504. doi: 10.1038/nrneph.2015.116. Epub 2015 Jul 14. Nat Rev Nephrol. 2015. PMID: 26168739 No abstract available.
Similar articles
-
Chronicity following ischaemia-reperfusion injury depends on tubular-macrophage crosstalk involving two tubular cell-derived CSF-1R activators: CSF-1 and IL-34.Nephrol Dial Transplant. 2016 Sep;31(9):1409-16. doi: 10.1093/ndt/gfw026. Epub 2016 Mar 24. Nephrol Dial Transplant. 2016. PMID: 27190368 Review.
-
Acute kidney injury: IL-34 promotes persistent ischaemia-induced AKI.Nat Rev Nephrol. 2015 Sep;11(9):504. doi: 10.1038/nrneph.2015.116. Epub 2015 Jul 14. Nat Rev Nephrol. 2015. PMID: 26168739 No abstract available.
-
C-reactive protein promotes acute kidney injury by impairing G1/S-dependent tubular epithelium cell regeneration.Clin Sci (Lond). 2014 May;126(9):645-59. doi: 10.1042/CS20130471. Clin Sci (Lond). 2014. PMID: 24206243
-
Trib1 Contributes to Recovery From Ischemia/Reperfusion-Induced Acute Kidney Injury by Regulating the Polarization of Renal Macrophages.Front Immunol. 2020 Mar 20;11:473. doi: 10.3389/fimmu.2020.00473. eCollection 2020. Front Immunol. 2020. PMID: 32265926 Free PMC article.
-
Metabolic Flexibility and Innate Immunity in Renal Ischemia Reperfusion Injury: The Fine Balance Between Adaptive Repair and Tissue Degeneration.Front Immunol. 2020 Jul 7;11:1346. doi: 10.3389/fimmu.2020.01346. eCollection 2020. Front Immunol. 2020. PMID: 32733450 Free PMC article. Review.
Cited by
-
Histone lysine crotonylation during acute kidney injury in mice.Dis Model Mech. 2016 Jun 1;9(6):633-45. doi: 10.1242/dmm.024455. Epub 2016 Apr 28. Dis Model Mech. 2016. PMID: 27125278 Free PMC article.
-
Cellular and Molecular Mechanisms of Intestinal Fibrosis.Gut Liver. 2023 May 15;17(3):360-374. doi: 10.5009/gnl220045. Epub 2023 Mar 10. Gut Liver. 2023. PMID: 36896620 Free PMC article. Review.
-
Single-cell transcriptomics and chromatin accessibility profiling elucidate the kidney-protective mechanism of mineralocorticoid receptor antagonists.J Clin Invest. 2024 Jan 2;134(1):e157165. doi: 10.1172/JCI157165. J Clin Invest. 2024. PMID: 37906287 Free PMC article.
-
Reno-protective effect of IL-34 inhibition on cisplatin-induced nephrotoxicity in mice.PLoS One. 2021 Jan 11;16(1):e0245340. doi: 10.1371/journal.pone.0245340. eCollection 2021. PLoS One. 2021. PMID: 33428678 Free PMC article.
-
Fate decision of satellite cell differentiation and self-renewal by miR-31-IL34 axis.Cell Death Differ. 2020 Mar;27(3):949-965. doi: 10.1038/s41418-019-0390-x. Epub 2019 Jul 22. Cell Death Differ. 2020. PMID: 31332295 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

