Disturbed Flow Induces Autophagy, but Impairs Autophagic Flux to Perturb Mitochondrial Homeostasis
- PMID: 26120766
- PMCID: PMC4657520
- DOI: 10.1089/ars.2014.5896
Disturbed Flow Induces Autophagy, but Impairs Autophagic Flux to Perturb Mitochondrial Homeostasis
Abstract
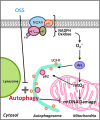
Aim: Temporal and spatial variations in shear stress are intimately linked with vascular metabolic effects. Autophagy is tightly regulated in intracellular bulk degradation/recycling system for maintaining cellular homeostasis. We postulated that disturbed flow modulates autophagy with an implication in mitochondrial superoxide (mtO2(•-)) production.
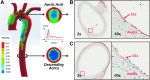
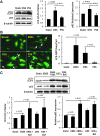
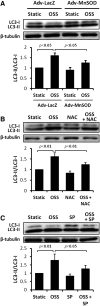
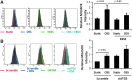
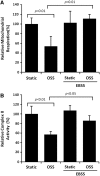
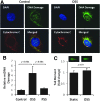
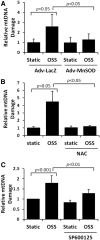
Results: In the disturbed flow or oscillatory shear stress (OSS)-exposed aortic arch, we observed prominent staining of p62, a reverse marker of autophagic flux, whereas in the pulsatile shear stress (PSS)-exposed descending aorta, p62 was attenuated. OSS significantly increased (i) microtubule-associated protein light chain 3 (LC3) II to I ratios in human aortic endothelial cells, (ii) autophagosome formation as quantified by green fluorescent protein (GFP)-LC3 dots per cell, and (iii) p62 protein levels, whereas manganese superoxide dismutase (MnSOD) overexpression by recombinant adenovirus, N-acetyl cysteine treatment, or c-Jun N-terminal kinase (JNK) inhibition reduced OSS-mediated LC3-II/LC3-I ratios and mitochondrial DNA damage. Introducing bafilomycin to Earle's balanced salt solution or to OSS condition incrementally increased both LC3-II/LC3-I ratios and p62 levels, implicating impaired autophagic flux. In the OSS-exposed aortic arch, both anti-phospho-JNK and anti-8-hydroxy-2'-deoxyguanosine (8-OHdG) staining for DNA damage were prominent, whereas in the PSS-exposed descending aorta, the staining was nearly absent. Knockdown of ATG5 with siRNA increased OSS-mediated mtO2(•-), whereas starvation or rapamycin-induced autophagy reduced OSS-mediated mtO2(•-), mitochondrial respiration, and complex II activity.
Innovation: Disturbed flow-mediated oxidative stress and JNK activation induce autophagy.
Conclusion: OSS impairs autophagic flux to interfere with mitochondrial homeostasis. Antioxid. Redox Signal. 23, 1207-1219.
Figures

Similar articles
-
Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling.Antioxid Redox Signal. 2011 Sep 1;15(5):1379-88. doi: 10.1089/ars.2010.3645. Epub 2011 Apr 14. Antioxid Redox Signal. 2011. PMID: 20919940 Free PMC article.
-
Time-dependent dysregulation of autophagy: Implications in aging and mitochondrial homeostasis in the kidney proximal tubule.Autophagy. 2016 May 3;12(5):801-13. doi: 10.1080/15548627.2016.1159376. Epub 2016 Mar 17. Autophagy. 2016. PMID: 26986194 Free PMC article.
-
BIX-01294 induces autophagy-associated cell death via EHMT2/G9a dysfunction and intracellular reactive oxygen species production.Autophagy. 2013 Dec;9(12):2126-39. doi: 10.4161/auto.26308. Autophagy. 2013. PMID: 24322755
-
Blood flow modulation of vascular dynamics.Curr Opin Lipidol. 2015 Oct;26(5):376-83. doi: 10.1097/MOL.0000000000000218. Curr Opin Lipidol. 2015. PMID: 26218416 Free PMC article. Review.
-
ATGs: Scaffolds for MAPK/ERK signaling.Autophagy. 2014 Mar;10(3):535-7. doi: 10.4161/auto.27642. Epub 2014 Jan 7. Autophagy. 2014. PMID: 24412893 Free PMC article. Review.
Cited by
-
Advanced microscopy to elucidate cardiovascular injury and regeneration: 4D light-sheet imaging.Prog Biophys Mol Biol. 2018 Oct;138:105-115. doi: 10.1016/j.pbiomolbio.2018.05.003. Epub 2018 May 9. Prog Biophys Mol Biol. 2018. PMID: 29752956 Free PMC article. Review.
-
Role of Flow-Sensitive Endothelial Genes in Atherosclerosis and Antiatherogenic Therapeutics Development.J Cardiovasc Transl Res. 2024 Jun;17(3):609-623. doi: 10.1007/s12265-023-10463-w. Epub 2023 Nov 27. J Cardiovasc Transl Res. 2024. PMID: 38010480 Review.
-
Elevated arterial shear rate increases indexes of endothelial cell autophagy and nitric oxide synthase activation in humans.Am J Physiol Heart Circ Physiol. 2019 Jan 1;316(1):H106-H112. doi: 10.1152/ajpheart.00561.2018. Epub 2018 Nov 9. Am J Physiol Heart Circ Physiol. 2019. PMID: 30412436 Free PMC article.
-
Autophagy is required for endothelial cell alignment and atheroprotection under physiological blood flow.Proc Natl Acad Sci U S A. 2017 Oct 10;114(41):E8675-E8684. doi: 10.1073/pnas.1702223114. Epub 2017 Sep 25. Proc Natl Acad Sci U S A. 2017. PMID: 28973855 Free PMC article.
-
Healthy versus Unhealthy Adipose Tissue Expansion: the Role of Exercise.J Obes Metab Syndr. 2022 Mar 30;31(1):37-50. doi: 10.7570/jomes21096. J Obes Metab Syndr. 2022. PMID: 35283364 Free PMC article. Review.
References
-
- Alemu EA, Lamark T, Torgersen KM, Birgisdottir AB, Larsen KB, Jain A, Olsvik H, Overvatn A, Kirkin V, and Johansen T. ATG8 family proteins act as scaffolds for assembly of the ULK complex: sequence requirements for LC3-interacting region (LIR) motifs. J Biol Chem 287: 39275–39290, 2012 - PMC - PubMed
-
- Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, and Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation 106: 544–549, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

