Connexin and pannexin signaling pathways, an architectural blueprint for CNS physiology and pathology?
- PMID: 26118660
- PMCID: PMC11113968
- DOI: 10.1007/s00018-015-1962-7
Connexin and pannexin signaling pathways, an architectural blueprint for CNS physiology and pathology?
Abstract
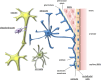
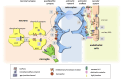
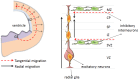
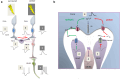
The central nervous system (CNS) is composed of a highly heterogeneous population of cells. Dynamic interactions between different compartments (neuronal, glial, and vascular systems) drive CNS function and allow to integrate and process information as well as to respond accordingly. Communication within this functional unit, coined the neuro-glio-vascular unit (NGVU), typically relies on two main mechanisms: direct cell-cell coupling via gap junction channels (GJCs) and paracrine communication via the extracellular compartment, two routes to which channels composed of transmembrane connexin (Cx) or pannexin (Panx) proteins can contribute. Multiple isoforms of both protein families are present in the CNS and each CNS cell type is characterized by a unique Cx/Panx portfolio. Over the last two decades, research has uncovered a multilevel platform via which Cxs and Panxs can influence different cellular functions within a tissue: (1) Cx GJCs enable a direct cell-cell communication of small molecules, (2) Cx hemichannels and Panx channels can contribute to autocrine/paracrine signaling pathways, and (3) different structural domains of these proteins allow for channel-independent functions, such as cell-cell adhesion, interactions with the cytoskeleton, and the activation of intracellular signaling pathways. In this paper, we discuss current knowledge on their multifaceted contribution to brain development and to specific processes in the NGVU, including synaptic transmission and plasticity, glial signaling, vasomotor control, and blood-brain barrier integrity in the mature CNS. By highlighting both physiological and pathological conditions, it becomes evident that Cxs and Panxs can play a dual role in the CNS and that an accurate fine-tuning of each signaling mechanism is crucial for normal CNS physiology.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest. C.R.G is a founding scientist of CoDa Therapeutics, Inc. which has intellectual property related to connexin channel modulation for therapeutic purposes.
Figures

Similar articles
-
Gap junction channels and hemichannels in the CNS: regulation by signaling molecules.Neuropharmacology. 2013 Dec;75:567-82. doi: 10.1016/j.neuropharm.2013.02.020. Epub 2013 Mar 7. Neuropharmacology. 2013. PMID: 23499663 Review.
-
Connexins and pannexins: At the junction of neuro-glial homeostasis & disease.J Neurosci Res. 2018 Jan;96(1):31-44. doi: 10.1002/jnr.24088. Epub 2017 Jun 5. J Neurosci Res. 2018. PMID: 28580666 Free PMC article. Review.
-
Connexin Channels at the Glio-Vascular Interface: Gatekeepers of the Brain.Neurochem Res. 2017 Sep;42(9):2519-2536. doi: 10.1007/s11064-017-2313-x. Epub 2017 Jun 20. Neurochem Res. 2017. PMID: 28634726 Review.
-
Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles.Front Pharmacol. 2013 Jul 17;4:88. doi: 10.3389/fphar.2013.00088. eCollection 2013. Front Pharmacol. 2013. PMID: 23882216 Free PMC article.
-
Differentiating connexin hemichannels and pannexin channels in cellular ATP release.FEBS Lett. 2014 Apr 17;588(8):1379-88. doi: 10.1016/j.febslet.2014.02.004. Epub 2014 Feb 15. FEBS Lett. 2014. PMID: 24548565 Free PMC article. Review.
Cited by
-
Connexin43 Region 266-283, via Src Inhibition, Reduces Neural Progenitor Cell Proliferation Promoted by EGF and FGF-2 and Increases Astrocytic Differentiation.Int J Mol Sci. 2020 Nov 23;21(22):8852. doi: 10.3390/ijms21228852. Int J Mol Sci. 2020. PMID: 33238452 Free PMC article.
-
Astrocytic responses to high glucose impair barrier formation in cerebral microvessel endothelial cells.Am J Physiol Regul Integr Comp Physiol. 2022 Jun 1;322(6):R571-R580. doi: 10.1152/ajpregu.00315.2020. Epub 2022 Apr 12. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 35412389 Free PMC article.
-
The Neuroglial Dialog Between Cannabinoids and Hemichannels.Front Mol Neurosci. 2018 Mar 20;11:79. doi: 10.3389/fnmol.2018.00079. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29662436 Free PMC article.
-
Regulation of Connexins Expression Levels by MicroRNAs, an Update.Front Physiol. 2016 Nov 25;7:558. doi: 10.3389/fphys.2016.00558. eCollection 2016. Front Physiol. 2016. PMID: 27932990 Free PMC article. Review.
-
Tonabersat Prevents Inflammatory Damage in the Central Nervous System by Blocking Connexin43 Hemichannels.Neurotherapeutics. 2017 Oct;14(4):1148-1165. doi: 10.1007/s13311-017-0536-9. Neurotherapeutics. 2017. PMID: 28560708 Free PMC article.
References
-
- Allen NJ, Barres BA. Neuroscience: glia—more than just brain glue. Nature. 2009;457(7230):675–677. - PubMed
-
- Ben Achour S, Pascual O. Glia: the many ways to modulate synaptic plasticity. Neurochem Int. 2010;57(4):440–445. - PubMed
-
- Perea G, Araque A. GLIA modulates synaptic transmission. Brain Res Rev. 2010;63(1–2):93–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

