Secretion of Fc-amidated peptide fusion proteins by Chinese hamster ovary cells
- PMID: 26116580
- PMCID: PMC4482046
- DOI: 10.1186/s12896-015-0173-5
Secretion of Fc-amidated peptide fusion proteins by Chinese hamster ovary cells
Abstract
Background: The therapeutic use of α-amidated peptides (e.g. calcitonin, glucagon-like peptide) has increased dramatically, but there are major impediments to wider use of such peptides. Larger peptides are expensive to synthesize, and short plasma half-lives frequently limit the clinical circumstances in which the peptides would be useful. Both problems are potentially solved by producing peptides as fusions with the Fc region of human immunoglobulin.
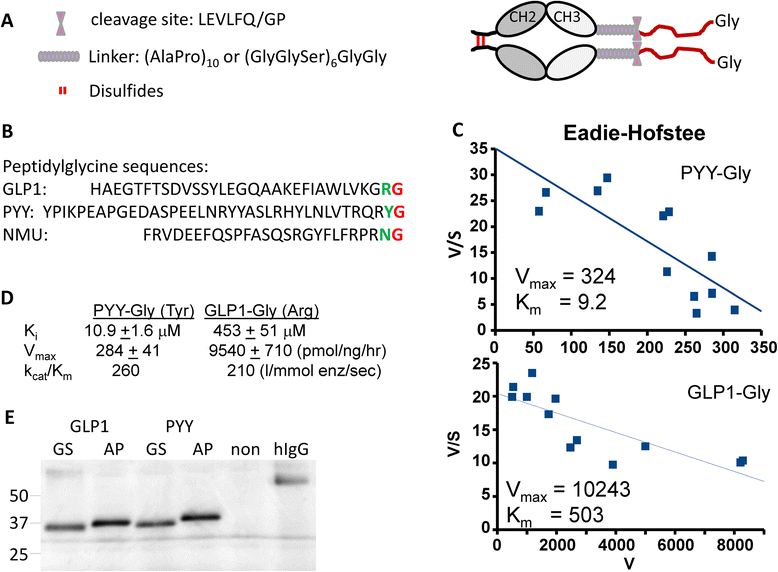
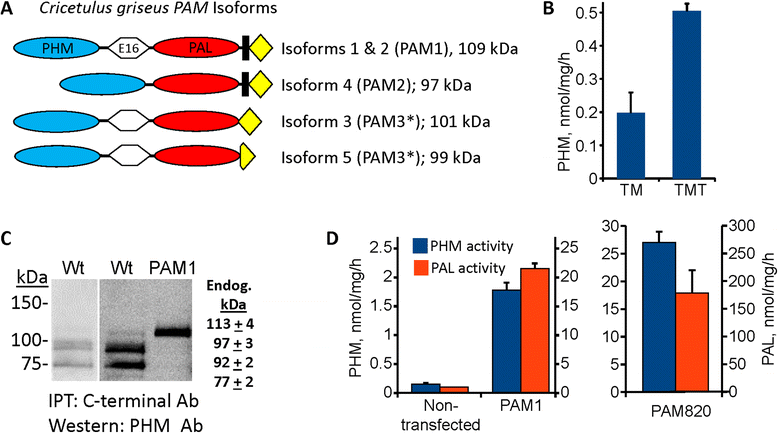
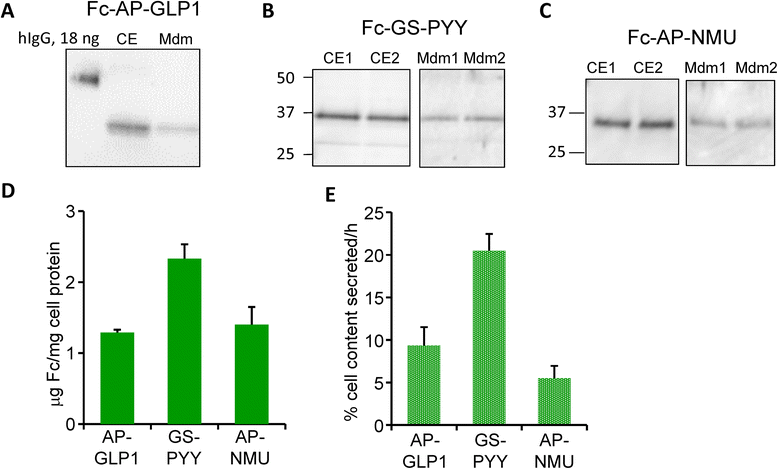
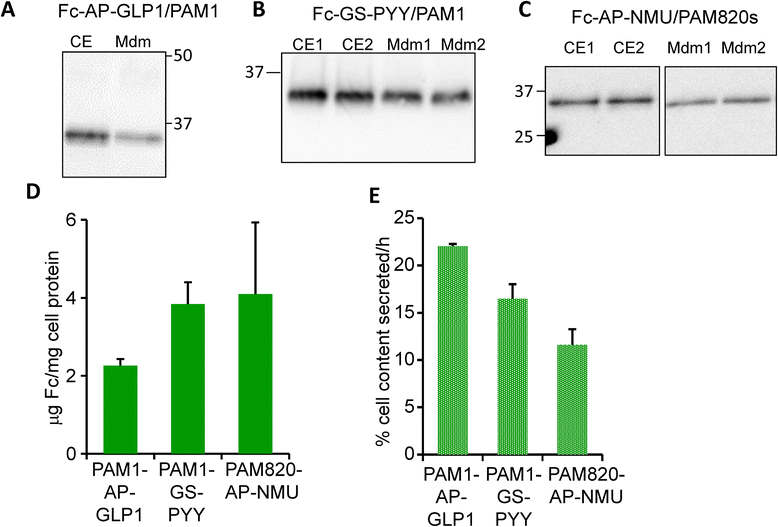
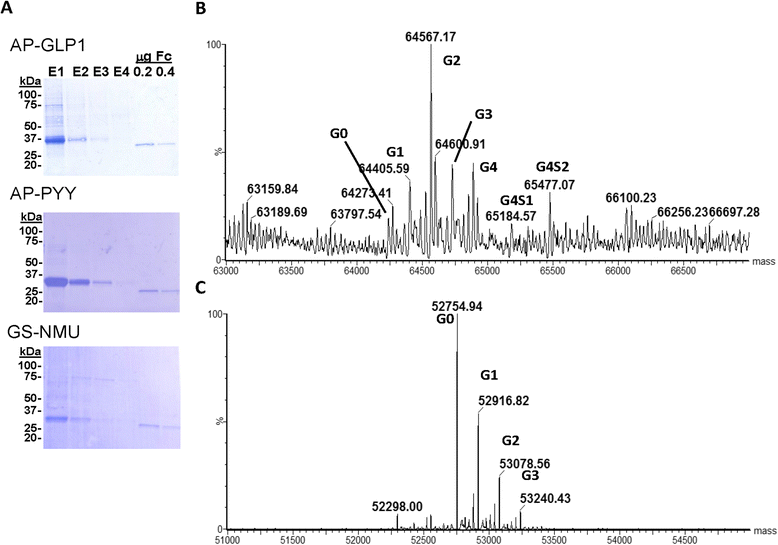
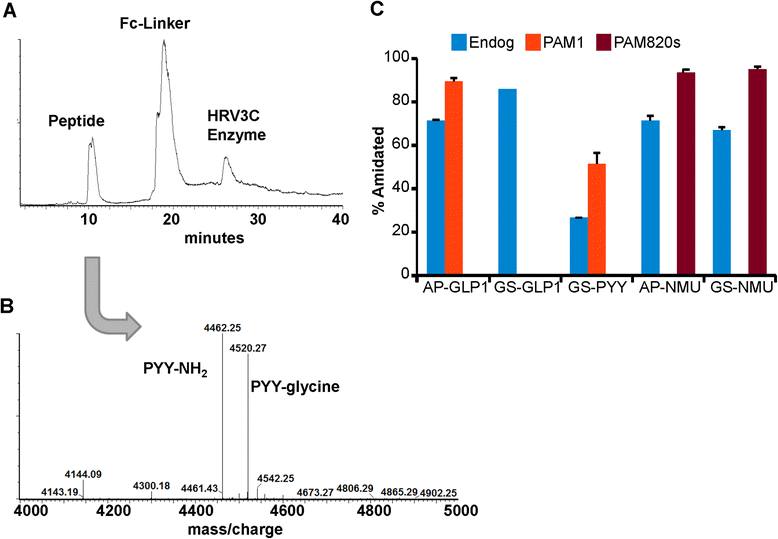
Methods: Glucagon-like peptide 1 (GLP1), peptide YY (PYY) and neuromedin U (NMU) were expressed and purified from stable CHO lines; since the α-amide group is essential for full biological potency of many peptides, Fc-fusion peptides were expressed in CHO lines stably expressing the α-amidating enzyme, peptidylglycine α-amidating monooxygenase (PAM: EC 1.14.17.3). Purified fusion proteins were analyzed intact and after HRV3C rhinovirus protease cleavage, at a site in the linker separating the Fc region from the peptide, by mass spectrometry and amide-specific immunoassays.
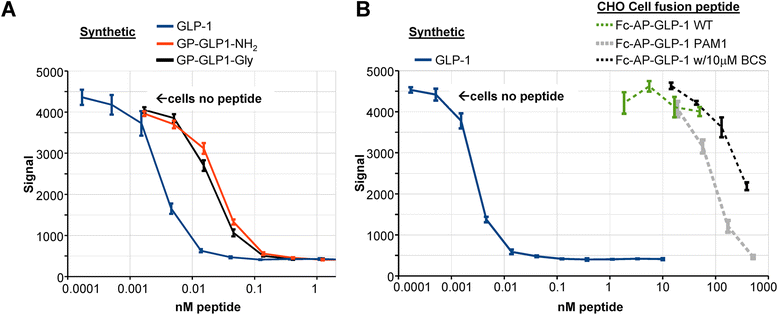
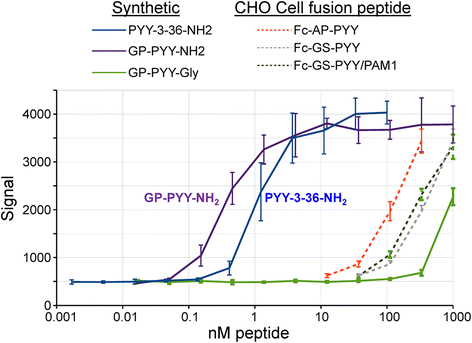
Results: The Fc fusions were expressed at 1-2.5 μg/mg cell protein and secreted at 5-20% of cell content per hour, in a peptide-specific manner. CHO cells express measurable endogenous PAM activity, amidating 25% of Fc-PYY and almost 90% of Fc-GLP1. Expression of exogenous PAM increased the level of peptide amidation to 50% of Fc-PYY and 95 % of Fc-NMU. The Fc-GLP1 fusions were 10,000-fold less active than synthetic GLP1 in a cell-receptor cyclic AMP-based assay, as expected since the amino terminal of GLP1 is essential for full biological activity. The Fc-PYY fusions were 100-fold less active than PYY-NH2 but 10-fold more active than non-amidated PYY-Gly.
Conclusions: This type of approach can be used for the production of stabilized α-amidated peptides aimed at clinical trials.
Figures
Similar articles
-
Optimizing production of Fc-amidated peptides by Chinese hamster ovary cells.BMC Biotechnol. 2015 Oct 16;15:95. doi: 10.1186/s12896-015-0210-4. BMC Biotechnol. 2015. PMID: 26475607 Free PMC article.
-
Effect of Bcl-xL overexpression on sialylation of Fc-fusion protein in recombinant Chinese hamster ovary cell cultures.Biotechnol Prog. 2015 Jul-Aug;31(4):1133-6. doi: 10.1002/btpr.2115. Epub 2015 Jun 26. Biotechnol Prog. 2015. PMID: 26018766
-
Expression of recombinant human bifunctional peptidylglycine α-amidating monooxygenase in CHO cells and its use for insulin analogue modification.Protein Expr Purif. 2016 Mar;119:102-9. doi: 10.1016/j.pep.2015.11.017. Epub 2015 Nov 22. Protein Expr Purif. 2016. PMID: 26614892
-
[Site-specific O-Glycosylation Analysis of Therapeutic Fc-fusion Protein by Mass Spectrometry].Yakugaku Zasshi. 2018;138(12):1483-1494. doi: 10.1248/yakushi.18-00020-2. Yakugaku Zasshi. 2018. PMID: 30504662 Review. Japanese.
-
Receptor-Fc fusion therapeutics, traps, and MIMETIBODY technology.Curr Opin Biotechnol. 2009 Dec;20(6):692-9. doi: 10.1016/j.copbio.2009.10.010. Epub 2009 Nov 4. Curr Opin Biotechnol. 2009. PMID: 19889530 Review.
Cited by
-
Generation of new peptide-Fc fusion proteins that mediate antibody-dependent cellular cytotoxicity against different types of cancer cells.Mol Ther Methods Clin Dev. 2015 Nov 4;2:15043. doi: 10.1038/mtm.2015.43. eCollection 2015. Mol Ther Methods Clin Dev. 2015. PMID: 26605373 Free PMC article.
-
The effect of fatty diacid acylation of human PYY3-36 on Y2 receptor potency and half-life in minipigs.Sci Rep. 2021 Oct 27;11(1):21179. doi: 10.1038/s41598-021-00654-3. Sci Rep. 2021. PMID: 34707178 Free PMC article.
-
Optimizing production of Fc-amidated peptides by Chinese hamster ovary cells.BMC Biotechnol. 2015 Oct 16;15:95. doi: 10.1186/s12896-015-0210-4. BMC Biotechnol. 2015. PMID: 26475607 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

