Loss of L-FABP, SCP-2/SCP-x, or both induces hepatic lipid accumulation in female mice
- PMID: 26116377
- PMCID: PMC4523233
- DOI: 10.1016/j.abb.2015.06.009
Loss of L-FABP, SCP-2/SCP-x, or both induces hepatic lipid accumulation in female mice
Abstract
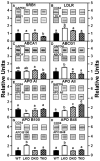
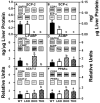
Although roles for both sterol carrier protein-2/sterol carrier protein-x (SCP-2/SCP-x) and liver fatty acid binding protein (L-FABP) have been proposed in hepatic lipid accumulation, individually ablating these genes has been complicated by concomitant alterations in the other gene product(s). For example, ablating SCP2/SCP-x induces upregulation of L-FABP in female mice. Therefore, the impact of ablating SCP-2/SCP-x (DKO) or L-FABP (LKO) individually or both together (TKO) was examined in female mice. Loss of SCP-2/SCP-x (DKO, TKO) more so than loss of L-FABP alone (LKO) increased hepatic total lipid and total cholesterol content, especially cholesteryl ester. Hepatic accumulation of nonesterified long chain fatty acids (LCFA) and phospholipids occurred only in DKO and TKO mice. Loss of SCP-2/SCP-x (DKO, TKO) increased serum total lipid primarily by increasing triglycerides. Altered hepatic level of proteins involved in cholesterol uptake, efflux, and/or secretion was observed, but did not compensate for the loss of L-FABP, SCP-2/SCP-x or both. However, synergistic responses were not seen with the combinatorial knock out animals-suggesting that inhibiting SCP-2/SCP-x is more correlative with hepatic dysfunction than L-FABP. The DKO- and TKO-induced hepatic accumulation of cholesterol and long chain fatty acids shared significant phenotypic similarities with non-alcoholic fatty liver disease (NAFLD).
Keywords: Gene ablation; L-FABP; SCP-2; SCP-x.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Ablating L-FABP in SCP-2/SCP-x null mice impairs bile acid metabolism and biliary HDL-cholesterol secretion.Am J Physiol Gastrointest Liver Physiol. 2014 Dec 1;307(11):G1130-43. doi: 10.1152/ajpgi.00209.2014. Epub 2014 Oct 2. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 25277800 Free PMC article.
-
Relative contributions of L-FABP, SCP-2/SCP-x, or both to hepatic biliary phenotype of female mice.Arch Biochem Biophys. 2015 Dec 15;588:25-32. doi: 10.1016/j.abb.2015.10.018. Epub 2015 Nov 2. Arch Biochem Biophys. 2015. PMID: 26541319 Free PMC article.
-
Ablating both Fabp1 and Scp2/Scpx (TKO) induces hepatic phospholipid and cholesterol accumulation in high fat-fed mice.Biochim Biophys Acta Mol Cell Biol Lipids. 2018 Mar;1863(3):323-338. doi: 10.1016/j.bbalip.2017.12.013. Epub 2018 Jan 4. Biochim Biophys Acta Mol Cell Biol Lipids. 2018. PMID: 29307784 Free PMC article.
-
Sterol carrier protein 2 in lipid metabolism and non-alcoholic fatty liver disease: Pathophysiology, molecular biology, and potential clinical implications.Metabolism. 2022 Jun;131:155180. doi: 10.1016/j.metabol.2022.155180. Epub 2022 Mar 17. Metabolism. 2022. PMID: 35311663 Review.
-
Liver fatty acid-binding protein and obesity.J Nutr Biochem. 2010 Nov;21(11):1015-32. doi: 10.1016/j.jnutbio.2010.01.005. J Nutr Biochem. 2010. PMID: 20537520 Free PMC article. Review.
Cited by
-
Effect of Fabp1/Scp-2/Scp-x Ablation on Whole Body and Hepatic Phenotype of Phytol-Fed Male Mice.Lipids. 2017 May;52(5):385-397. doi: 10.1007/s11745-017-4249-y. Epub 2017 Apr 5. Lipids. 2017. PMID: 28382456 Free PMC article.
-
An Overview of Lipid Metabolism and Nonalcoholic Fatty Liver Disease.Biomed Res Int. 2020 Jul 18;2020:4020249. doi: 10.1155/2020/4020249. eCollection 2020. Biomed Res Int. 2020. PMID: 32733940 Free PMC article. Review.
-
SCP2-mediated cholesterol membrane trafficking promotes the growth of pituitary adenomas via Hedgehog signaling activation.J Exp Clin Cancer Res. 2019 Sep 13;38(1):404. doi: 10.1186/s13046-019-1411-9. J Exp Clin Cancer Res. 2019. PMID: 31519191 Free PMC article.
-
Lrp6 Genotype affects Individual Susceptibility to Nonalcoholic Fatty Liver Disease and Silibinin Therapeutic Response via Wnt/β-catenin-Cyp2e1 Signaling.Int J Biol Sci. 2021 Sep 21;17(14):3936-3953. doi: 10.7150/ijbs.63732. eCollection 2021. Int J Biol Sci. 2021. PMID: 34671210 Free PMC article.
-
Uridine Metabolism and Its Role in Glucose, Lipid, and Amino Acid Homeostasis.Biomed Res Int. 2020 Apr 14;2020:7091718. doi: 10.1155/2020/7091718. eCollection 2020. Biomed Res Int. 2020. PMID: 32382566 Free PMC article. Review.
References
-
- Gariani K, Philippe J, Jornayvaz FR. Non-alcoholic liver disease and insulin resistance: from bench to bedside. Diabetes and Metabolism. 2013;39:16–26. - PubMed
-
- Tailleux A, Wouters K, Staels B. Role of PPARs in NAFLD: potential therapeutic targets. Biochim Biophys Acta. 2012;1821:809–818. - PubMed
-
- Paz-Filho G, Mastronardi CA, Parker BJ, Khan A, Inserra A, Matthaei KI, Erhart-Bornstein M, Bornstein S, Wong M-L, Licinio J. Molecular pathways involved in the improvement of nonalcoholic fatty liver disease. J Mol Endocrinol. 2013;51:167–179. - PubMed
-
- Vluggens A, Reddy JK. Nuclear receptors and transcription factors in the development of fatty liver disease. Curr Drug Metabolism. 2012;13:1422–1435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

