Comparative Analysis of Apicoplast-Targeted Protein Extension Lengths in Apicomplexan Parasites
- PMID: 26114107
- PMCID: PMC4465681
- DOI: 10.1155/2015/452958
Comparative Analysis of Apicoplast-Targeted Protein Extension Lengths in Apicomplexan Parasites
Abstract
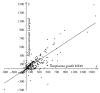
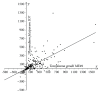
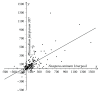
In general, the mechanism of protein translocation through the apicoplast membrane requires a specific extension of a functionally important region of the apicoplast-targeted proteins. The corresponding signal peptides were detected in many apicomplexans but not in the majority of apicoplast-targeted proteins in Toxoplasma gondii. In T. gondii signal peptides are either much diverged or their extension region is processed, which in either case makes the situation different from other studied apicomplexans. We propose a statistic method to compare extensions of the functionally important regions of apicoplast-targeted proteins. More specifically, we provide a comparison of extension lengths of orthologous apicoplast-targeted proteins in apicomplexan parasites. We focus on results obtained for the model species T. gondii, Neospora caninum, and Plasmodium falciparum. With our method, cross species comparisons demonstrate that, in average, apicoplast-targeted protein extensions in T. gondii are 1.5-fold longer than in N. caninum and 2-fold longer than in P. falciparum. Extensions in P. falciparum less than 87 residues in size are longer than the corresponding extensions in N. caninum and, reversely, are shorter if they exceed 88 residues.
Figures

Similar articles
-
Targeting of a Transporter to the Outer Apicoplast Membrane in the Human Malaria Parasite Plasmodium falciparum.PLoS One. 2016 Jul 21;11(7):e0159603. doi: 10.1371/journal.pone.0159603. eCollection 2016. PLoS One. 2016. PMID: 27442138 Free PMC article.
-
Autophagy-Related Protein ATG18 Regulates Apicoplast Biogenesis in Apicomplexan Parasites.mBio. 2017 Oct 31;8(5):e01468-17. doi: 10.1128/mBio.01468-17. mBio. 2017. PMID: 29089429 Free PMC article.
-
Protein Traffic to the Plasmodium falciparum apicoplast: evidence for a sorting branch point at the Golgi.Traffic. 2014 Dec;15(12):1290-304. doi: 10.1111/tra.12226. Epub 2014 Oct 15. Traffic. 2014. PMID: 25264207
-
Phylogeny and evolution of apicoplasts and apicomplexan parasites.Parasitol Int. 2015 Jun;64(3):254-9. doi: 10.1016/j.parint.2014.10.005. Epub 2014 Oct 14. Parasitol Int. 2015. PMID: 25451217 Review.
-
Replication and maintenance of the Plasmodium falciparum apicoplast genome.Mol Biochem Parasitol. 2016 Aug;208(2):56-64. doi: 10.1016/j.molbiopara.2016.06.006. Epub 2016 Jun 20. Mol Biochem Parasitol. 2016. PMID: 27338018 Review.
Cited by
-
A method for identification of highly conserved elements and evolutionary analysis of superphylum Alveolata.BMC Bioinformatics. 2016 Sep 20;17:385. doi: 10.1186/s12859-016-1257-5. BMC Bioinformatics. 2016. PMID: 27645252 Free PMC article.
-
Anti-malarial Drug Design by Targeting Apicoplasts: New Perspectives.J Pharmacopuncture. 2016 Mar;19(1):7-15. doi: 10.3831/KPI.2016.19.001. J Pharmacopuncture. 2016. PMID: 27280044 Free PMC article. Review.
-
Protein length distribution is remarkably uniform across the tree of life.Genome Biol. 2023 Jun 8;24(1):135. doi: 10.1186/s13059-023-02973-2. Genome Biol. 2023. PMID: 37291671 Free PMC article.
-
Optimal Growth Temperature and Intergenic Distances in Bacteria, Archaea, and Plastids of Rhodophytic Branch.Biomed Res Int. 2020 Jan 18;2020:3465380. doi: 10.1155/2020/3465380. eCollection 2020. Biomed Res Int. 2020. PMID: 32025518 Free PMC article.
-
Heterologous expression in Toxoplasma gondii reveals a topogenic signal anchor in a Plasmodium apicoplast protein.FEBS Open Bio. 2018 Oct 22;8(11):1746-1762. doi: 10.1002/2211-5463.12527. eCollection 2018 Nov. FEBS Open Bio. 2018. PMID: 30410855 Free PMC article.
References
-
- Ermak T. N., Peregudova A. B., Shakhgil'dian V. I., Goncharov D. B. Cerebral toxoplasmosis in the pattern of secondary CNS involvements in HIV-infected patients in the Russian federation: clinical and diagnostic features. Meditsinskaia Parazitologiia i Parazitarnye Bolezni. 2013;(1):3–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

