Extracellular Vesicles Present in Human Ovarian Tumor Microenvironments Induce a Phosphatidylserine-Dependent Arrest in the T-cell Signaling Cascade
- PMID: 26112921
- PMCID: PMC4636911
- DOI: 10.1158/2326-6066.CIR-15-0086
Extracellular Vesicles Present in Human Ovarian Tumor Microenvironments Induce a Phosphatidylserine-Dependent Arrest in the T-cell Signaling Cascade
Abstract
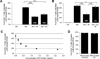
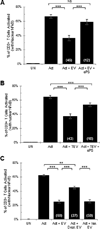
The identification of immunosuppressive factors within human tumor microenvironments, and the ability to block these factors, would be expected to enhance patients' antitumor immune responses. We previously established that an unidentified factor, or factors, present in ovarian tumor ascites fluids reversibly inhibited the activation of T cells by arresting the T-cell signaling cascade. Ultracentrifugation of the tumor ascites fluid has now revealed a pellet that contains small extracellular vesicles (EV) with an average diameter of 80 nm. The T-cell arrest was determined to be causally linked to phosphatidylserine (PS) that is present on the outer leaflet of the vesicle bilayer, as a depletion of PS-expressing EV or a blockade of PS with anti-PS antibody significantly inhibits the vesicle-induced signaling arrest. The inhibitory EV were also isolated from solid tumor tissues. The presence of immunosuppressive vesicles in the microenvironments of ovarian tumors and our ability to block their inhibition of T-cell function represent a potential therapeutic target for patients with ovarian cancer.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures




Similar articles
-
Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients.Cancer Immunol Immunother. 2015 Mar;64(3):337-47. doi: 10.1007/s00262-014-1636-6. Epub 2014 Nov 22. Cancer Immunol Immunother. 2015. PMID: 25416072 Free PMC article.
-
Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma.Nat Commun. 2019 Jul 5;10(1):3000. doi: 10.1038/s41467-019-10979-3. Nat Commun. 2019. PMID: 31278254 Free PMC article.
-
Human ovarian tumor ascites fluids rapidly and reversibly inhibit T cell receptor-induced NF-κB and NFAT signaling in tumor-associated T cells.Cancer Immun. 2013 Jul 15;13:14. Print 2013. Cancer Immun. 2013. PMID: 23882159 Free PMC article.
-
Peripheral tolerance in CD8+ T cells.Cytokine. 2009 May;46(2):147-59. doi: 10.1016/j.cyto.2009.01.010. Epub 2009 Mar 5. Cytokine. 2009. PMID: 19268604 Review.
-
Inflammation and ovarian cancer--current views.Ginekol Pol. 2013 Apr;84(4):293-7. doi: 10.17772/gp/1579. Ginekol Pol. 2013. PMID: 23700863 Review.
Cited by
-
An Immunosuppressive Effect of Melanoma-derived Exosomes on NY-ESO-1 Antigen-specific Human CD8+ T Cells is Dependent on IL-10 and Independent of BRAFV600E Mutation in Melanoma Cell Lines.Immunol Invest. 2020 Oct;49(7):744-757. doi: 10.1080/08820139.2020.1803353. Epub 2020 Aug 17. Immunol Invest. 2020. PMID: 32799717 Free PMC article.
-
Role of exosomes in the immune microenvironment of ovarian cancer.Oncol Lett. 2021 May;21(5):377. doi: 10.3892/ol.2021.12638. Epub 2021 Mar 15. Oncol Lett. 2021. PMID: 33777201 Free PMC article. Review.
-
Exosomes Associated with Human Ovarian Tumors Harbor a Reversible Checkpoint of T-cell Responses.Cancer Immunol Res. 2018 Feb;6(2):236-247. doi: 10.1158/2326-6066.CIR-17-0113. Epub 2018 Jan 4. Cancer Immunol Res. 2018. PMID: 29301753 Free PMC article.
-
Phosphatidylserine Is Not Just a Cleanup Crew but Also a Well-Meaning Teacher.J Pharm Sci. 2018 Aug;107(8):2048-2054. doi: 10.1016/j.xphs.2018.03.027. Epub 2018 Apr 9. J Pharm Sci. 2018. PMID: 29649469 Free PMC article.
-
Phosphatidylserine-Liposomes Promote Tolerogenic Features on Dendritic Cells in Human Type 1 Diabetes by Apoptotic Mimicry.Front Immunol. 2018 Feb 14;9:253. doi: 10.3389/fimmu.2018.00253. eCollection 2018. Front Immunol. 2018. PMID: 29491866 Free PMC article.
References
-
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

