The MUTYH base excision repair gene protects against inflammation-associated colorectal carcinogenesis
- PMID: 26109431
- PMCID: PMC4637313
- DOI: 10.18632/oncotarget.4284
The MUTYH base excision repair gene protects against inflammation-associated colorectal carcinogenesis
Abstract
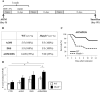
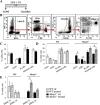
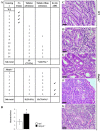
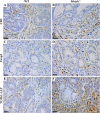
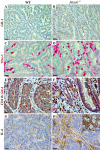
MUTYH DNA glycosylase removes mismatched adenine opposite 7, 8-dihydro-8-oxoguanine (8-oxoG), which is the major mutagenic lesion induced by oxidative stress. Biallelic mutations in MUTYH are associated with MUTYH-Associated polyposis (MAP) and increased risk in colorectal cancer (CRC). We investigated cancer susceptibility associated with MUTYH inactivation in a mouse model of inflammation-dependent carcinogenesis induced by azoxymethane (AOM) and dextran sulphate (DSS). Mutyh-/- mice were more sensitive than wild-type (WT) animals to AOM/DSS toxicity and accumulated DNA 8-oxoG in their gastrointestinal tract. AOM/DSS-induced colonic adenomas were significantly more numerous in Mutyh-/- than in WT animals, and frequently showed a tubulo-villous feature along with high-grade dysplasia and larger size lesions. This condition resulted in a greater propensity to develop adenocarcinomas. The colon of untreated Mutyh-/- mice expressed higher basal levels of pro-inflammatory cytokines GM-CSF and IFNγ, and treatment with AOM/DSS induced an early decrease in circulating CD4+ and CD8+ T lymphocytes and an increase in myeloid-derived suppressor cells (MDSCs). Adenomas from Mutyh-/- mice had a greater infiltrate of Foxp3+ T regulatory cells, granulocytes, macrophages, MDSCs and strong expression of TGF-β-latency-associated peptide and IL6. Our findings indicate that MUTYH loss is associated with an increase in CRC risk, which involves immunosuppression and altered inflammatory response. We propose that the AOM/DSS initiation/promotion protocol in Mutyh-/- mice provides a good model for MAP.
Keywords: DSS; MUTYH; azoxymethane; colorectal cancer; inflammation.
Conflict of interest statement
The authors declare that there are no competing financial interests in relation to the work described.
Figures
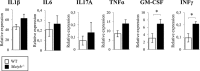
Similar articles
-
Upregulation of PD-1 follows tumour development in the AOM/DSS model of inflammation-induced colorectal cancer in mice.Immunology. 2019 Sep;158(1):35-46. doi: 10.1111/imm.13093. Immunology. 2019. PMID: 31429085 Free PMC article.
-
Prevention of azoxymethane/dextran sodium sulfate-induced mouse colon carcinogenesis by processed Aloe vera gel.Int Immunopharmacol. 2016 Nov;40:428-435. doi: 10.1016/j.intimp.2016.09.022. Epub 2016 Sep 30. Int Immunopharmacol. 2016. PMID: 27697726
-
Organomagnesium suppresses inflammation-associated colon carcinogenesis in male Crj: CD-1 mice.Carcinogenesis. 2013 Feb;34(2):361-9. doi: 10.1093/carcin/bgs348. Epub 2012 Nov 3. Carcinogenesis. 2013. PMID: 23125223
-
Repair of 8-oxoG:A mismatches by the MUTYH glycosylase: Mechanism, metals and medicine.Free Radic Biol Med. 2017 Jun;107:202-215. doi: 10.1016/j.freeradbiomed.2017.01.008. Epub 2017 Jan 10. Free Radic Biol Med. 2017. PMID: 28087410 Free PMC article. Review.
-
MUTYH-associated polyposis--from defect in base excision repair to clinical genetic testing.DNA Repair (Amst). 2007 Mar 1;6(3):274-9. doi: 10.1016/j.dnarep.2006.11.001. Epub 2006 Dec 11. DNA Repair (Amst). 2007. PMID: 17161978 Review.
Cited by
-
Relationship between MUTYH, OGG1 and BRCA1 mutations and mRNA expression in breast and ovarian cancer predisposition.Mol Clin Oncol. 2021 Jan;14(1):15. doi: 10.3892/mco.2020.2177. Epub 2020 Nov 26. Mol Clin Oncol. 2021. PMID: 33343895 Free PMC article.
-
Colorectal cancer development is affected by the ECM molecule EMILIN-2 hinging on macrophage polarization via the TLR-4/MyD88 pathway.J Exp Clin Cancer Res. 2022 Feb 11;41(1):60. doi: 10.1186/s13046-022-02271-y. J Exp Clin Cancer Res. 2022. PMID: 35148799 Free PMC article.
-
TNF-alpha and metalloproteases as key players in melanoma cells aggressiveness.J Exp Clin Cancer Res. 2018 Dec 28;37(1):326. doi: 10.1186/s13046-018-0982-1. J Exp Clin Cancer Res. 2018. PMID: 30591049 Free PMC article.
-
Association of a New Germline Variant in the MUTYH DNA Glycosylase Gene with Colorectal Adenoma Transformation into Malignancy.Iran Biomed J. 2019 Nov;23(6):412-22. doi: 10.29252/ibj.23.6.412. Epub 2019 May 20. Iran Biomed J. 2019. PMID: 31104418 Free PMC article.
-
Resolvin E1 Reduces Tumor Growth in a Xenograft Model of Lung Cancer.Am J Pathol. 2022 Oct;192(10):1470-1484. doi: 10.1016/j.ajpath.2022.07.004. Epub 2022 Aug 6. Am J Pathol. 2022. PMID: 35944728 Free PMC article.
References
-
- van Loon B, Markkanen E, Hübscher U. Oxygen as a friend and enemy: how to combat the mutational potential of 8-oxo-guanine. DNA Repair (Amst.) 2010;9:604–616. - PubMed
-
- Mazzei F, Viel A, Bignami M. Role of MUTYH in human cancer. Mutat Res. 2013;743–744:33–43. - PubMed
-
- Al-Tassan N, Chmiel NH, Maynard J, Fleming N, Livingston AL, Williams GT, Hodges AK, Davies DR, David SS, Sampson JR, Cheadle JP. Inherited variants of MYH associated with somatic G:C–>T:A mutations in colorectal tumors. Nat Genet. 2002;30:227–232. - PubMed
-
- Jones S, Emmerson P, Maynard J, Best JM, Jordan S, Williams GT, Sampson JR, Cheadle JP. Biallelic germline mutations in MYH predispose to multiple colorectal adenoma and somatic G:C–>T:A mutations. Hum Mol Genet. 2002;11:2961–2967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

