An eIF2α-binding motif in protein phosphatase 1 subunit GADD34 and its viral orthologs is required to promote dephosphorylation of eIF2α
- PMID: 26100893
- PMCID: PMC4500263
- DOI: 10.1073/pnas.1501557112
An eIF2α-binding motif in protein phosphatase 1 subunit GADD34 and its viral orthologs is required to promote dephosphorylation of eIF2α
Abstract
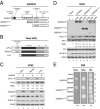
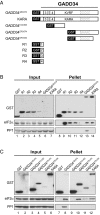
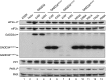
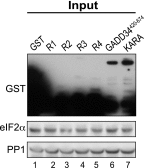
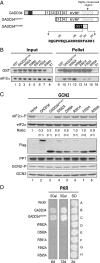
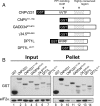
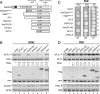
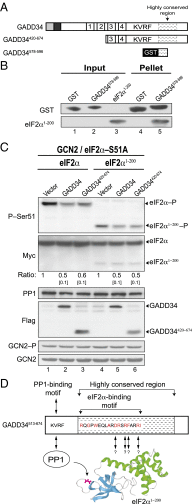
Transient protein synthesis inhibition, mediated by phosphorylation of the α subunit of eukaryotic translation initiation factor 2 (eIF2α), is an important protective mechanism cells use during stress conditions. Following relief of the stress, the growth arrest and DNA damage-inducible protein GADD34 associates with the broadly acting serine/threonine protein phosphatase 1 (PP1) to dephosphorylate eIF2α. Whereas the PP1-binding motif on GADD34 has been defined, it remains to be determined how GADD34 directs PP1 to specifically dephosphorylate eIF2α. In this report, we map a novel eIF2α-binding motif to the C terminus of GADD34 in a region distinct from where PP1 binds to GADD34. This motif is characterized by the consensus sequence Rx[Gnl]x(1-2)Wxxx[Arlv]x[Dn][Rg]xRFxx[Rlvk][Ivc], where capital letters are preferred and x is any residue. Point mutations altering the eIF2α-binding motif impair the ability of GADD34 to interact with eIF2α, promote eIF2α dephosphorylation, and suppress PKR toxicity in yeast. Interestingly, this eIF2α-docking motif is conserved among viral orthologs of GADD34, and is necessary for the proteins produced by African swine fever virus, Canarypox virus, and Herpes simplex virus to promote eIF2α dephosphorylation. Taken together, these data indicate that GADD34 and its viral orthologs direct specific dephosphorylation of eIF2α by interacting with both PP1 and eIF2α through independent binding motifs.
Keywords: CReP; DP71L; PKR; PP1; canarypox.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Protein phosphatase PP1/GLC7 interaction domain in yeast eIF2γ bypasses targeting subunit requirement for eIF2α dephosphorylation.Proc Natl Acad Sci U S A. 2014 Apr 8;111(14):E1344-53. doi: 10.1073/pnas.1400129111. Epub 2014 Mar 24. Proc Natl Acad Sci U S A. 2014. PMID: 24706853 Free PMC article.
-
ICP34.5 protein of herpes simplex virus facilitates the initiation of protein translation by bridging eukaryotic initiation factor 2alpha (eIF2alpha) and protein phosphatase 1.J Biol Chem. 2011 Jul 15;286(28):24785-92. doi: 10.1074/jbc.M111.232439. Epub 2011 May 26. J Biol Chem. 2011. PMID: 21622569 Free PMC article.
-
Identification of residues within the African swine fever virus DP71L protein required for dephosphorylation of translation initiation factor eIF2α and inhibiting activation of pro-apoptotic CHOP.Virology. 2017 Apr;504:107-113. doi: 10.1016/j.virol.2017.02.002. Epub 2017 Feb 9. Virology. 2017. PMID: 28189088 Free PMC article.
-
The PPP1R15 Family of eIF2-alpha Phosphatase Targeting Subunits (GADD34 and CReP).Int J Mol Sci. 2023 Dec 10;24(24):17321. doi: 10.3390/ijms242417321. Int J Mol Sci. 2023. PMID: 38139150 Free PMC article. Review.
-
An Overview of Methods for Detecting eIF2α Phosphorylation and the Integrated Stress Response.Methods Mol Biol. 2022;2428:3-18. doi: 10.1007/978-1-0716-1975-9_1. Methods Mol Biol. 2022. PMID: 35171470 Review.
Cited by
-
The role of host eIF2α in viral infection.Virol J. 2020 Jul 23;17(1):112. doi: 10.1186/s12985-020-01362-6. Virol J. 2020. PMID: 32703221 Free PMC article. Review.
-
The regulatory protein GADD34 inhibits TRAIL-induced apoptosis via TRAF6/ERK-dependent stabilization of myeloid cell leukemia 1 in liver cancer cells.J Biol Chem. 2019 Apr 12;294(15):5945-5955. doi: 10.1074/jbc.RA118.006029. Epub 2019 Feb 19. J Biol Chem. 2019. PMID: 30782845 Free PMC article.
-
Delineating the role of eIF2α in retinal degeneration.Cell Death Dis. 2019 May 28;10(6):409. doi: 10.1038/s41419-019-1641-y. Cell Death Dis. 2019. PMID: 31138784 Free PMC article.
-
Type I interferons and endoplasmic reticulum stress in health and disease.Int Rev Cell Mol Biol. 2020;350:63-118. doi: 10.1016/bs.ircmb.2019.10.004. Epub 2019 Nov 19. Int Rev Cell Mol Biol. 2020. PMID: 32138904 Free PMC article. Review.
-
Minding the message: tactics controlling RNA decay, modification, and translation in virus-infected cells.Genes Dev. 2022 Feb 1;36(3-4):108-132. doi: 10.1101/gad.349276.121. Genes Dev. 2022. PMID: 35193946 Free PMC article. Review.
References
-
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. - PubMed
-
- Dever TE, et al. Phosphorylation of initiation factor 2 α by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68(3):585–596. - PubMed
-
- Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev Biol. 2005;16(1):3–12. - PubMed
-
- Hinnebusch AG. Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor Lab Press; Plainview, NY: 2000. pp. 185–243.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

