Increased expression of NAPDH oxidase 4 in systemic sclerosis dermal fibroblasts: regulation by transforming growth factor β
- PMID: 26096997
- PMCID: PMC4581953
- DOI: 10.1002/art.39242
Increased expression of NAPDH oxidase 4 in systemic sclerosis dermal fibroblasts: regulation by transforming growth factor β
Abstract
Objective: Systemic sclerosis (SSc) is characterized by severe and often progressive fibrosis of the skin and multiple internal organs. The mechanisms responsible for these alterations remain obscure, although excessive reactive oxygen species (ROS)-mediated oxidative stress has been implicated. NOX-4 is 1 of 7 isoforms of NADPH oxidase responsible for the generation of ROS. The purpose of this study was to examine NOX-4 expression in skin and cultured dermal fibroblasts from SSc patients and to examine its regulation by transforming growth factor β1 (TGFβ1).
Methods: NOX-4 was assessed in normal and SSc skin by immunohistologic analysis and in normal and SSc cultured dermal fibroblasts by quantitative polymerase chain reaction analysis, fluorescence microscopy, and Western blotting. ROS levels were assessed by fluorescence measurement of H2 O2 production. Specific kinase inhibitors were used to study the TGFβ1 signaling involved in NOX-4 stimulation. NOX-4 inhibition/down-regulation was induced with a selective NOX-4 small-molecule inhibitor and NOX-4 small interfering RNA (siRNA).
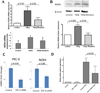
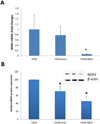
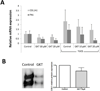
Results: In contrast with normal skin fibroblasts, those from SSc skin showed intense NOX-4 staining. Cultured SSc fibroblasts displayed increased NOX-4 expression. TGFβ1 caused potent NOX-4 protein and messenger RNA stimulation in normal and SSc fibroblasts, which was mediated by the protein kinase Cδ (PKCδ) and Smad2/3 pathways. NOX-4 knockdown in SSc fibroblasts reduced the production of ROS and lowered the expression of type I collagen.
Conclusion: NOX-4 expression and production were found to be constitutively elevated in SSc skin and cultured SSc dermal fibroblasts. TGFβ1 stimulated NOX-4 expression in normal and SSc fibroblasts through PKCδ and Smad2/3 signaling pathways. A small-molecule NOX-4 inhibitor decreased collagen and fibronectin production by normal and SSc fibroblasts, and NOX-4 siRNA knockdown reduced ROS and collagen production by SSc fibroblasts. These results demonstrate the involvement of NOX-4 in SSc-associated fibrosis and indicate NOX-4 inhibitors as novel therapeutic approaches for SSc.
© 2015, American College of Rheumatology.
Figures



Similar articles
-
Oxidative Stress Induced by Reactive Oxygen Species (ROS) and NADPH Oxidase 4 (NOX4) in the Pathogenesis of the Fibrotic Process in Systemic Sclerosis: A Promising Therapeutic Target.J Clin Med. 2021 Oct 19;10(20):4791. doi: 10.3390/jcm10204791. J Clin Med. 2021. PMID: 34682914 Free PMC article. Review.
-
A reactive oxygen species-mediated loop maintains increased expression of NADPH oxidases 2 and 4 in skin fibroblasts from patients with systemic sclerosis.Arthritis Rheumatol. 2015 Jun;67(6):1611-22. doi: 10.1002/art.39084. Arthritis Rheumatol. 2015. PMID: 25707572
-
Oxidative stress in scleroderma: maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway.Arthritis Rheum. 2001 Nov;44(11):2653-64. doi: 10.1002/1529-0131(200111)44:11<2653::aid-art445>3.0.co;2-1. Arthritis Rheum. 2001. PMID: 11710721
-
NADPH oxidase-2 is a key regulator of human dermal fibroblasts: a potential therapeutic strategy for the treatment of skin fibrosis.Exp Dermatol. 2014 Sep;23(9):639-44. doi: 10.1111/exd.12479. Exp Dermatol. 2014. PMID: 24981855
-
Regulation of connective tissue synthesis in systemic sclerosis.Int Rev Immunol. 1995;12(2-4):187-99. doi: 10.3109/08830189509056712. Int Rev Immunol. 1995. PMID: 7650421 Review.
Cited by
-
Development and validation of a new diagnostic prediction model of ENHO and NOX4 for early diagnosis of systemic sclerosis.Front Immunol. 2024 Jan 29;15:1273559. doi: 10.3389/fimmu.2024.1273559. eCollection 2024. Front Immunol. 2024. PMID: 38348042 Free PMC article.
-
Activin A-Mediated Regulation of XT-I in Human Skin Fibroblasts.Biomolecules. 2020 Apr 14;10(4):609. doi: 10.3390/biom10040609. Biomolecules. 2020. PMID: 32295230 Free PMC article.
-
Oxidative Stress and Epigenetics: miRNA Involvement in Rare Autoimmune Diseases.Antioxidants (Basel). 2023 Mar 25;12(4):800. doi: 10.3390/antiox12040800. Antioxidants (Basel). 2023. PMID: 37107175 Free PMC article. Review.
-
Oxidative Stress Induced by Reactive Oxygen Species (ROS) and NADPH Oxidase 4 (NOX4) in the Pathogenesis of the Fibrotic Process in Systemic Sclerosis: A Promising Therapeutic Target.J Clin Med. 2021 Oct 19;10(20):4791. doi: 10.3390/jcm10204791. J Clin Med. 2021. PMID: 34682914 Free PMC article. Review.
-
Endothelial to Mesenchymal Transition (EndoMT) in the Pathogenesis of Human Fibrotic Diseases.J Clin Med. 2016 Apr 11;5(4):45. doi: 10.3390/jcm5040045. J Clin Med. 2016. PMID: 27077889 Free PMC article. Review.
References
-
- Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140:37–50. - PubMed
-
- Katsumoto TR, Whitfield ML, Connolly MK. The pathogenesis of systemic sclerosis. Annual Rev Pathol. 2011;6:509–537. - PubMed
-
- Balbir-Gurman A, Braun-Moscovici Y. Scleroderma-new aspects in pathogenesis and treatment. Best Pract Res Clin Rheumatol. 2012;26:13–24. - PubMed
-
- Denton CP, Black CM, Abraham DJ. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2:134–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

