Adjuvants may reduce in vivo transfection levels for DNA vaccination in mice leading to reduced antigen-specific CD8+ T cell responses
- PMID: 26091088
- PMCID: PMC4635848
- DOI: 10.1080/21645515.2015.1047567
Adjuvants may reduce in vivo transfection levels for DNA vaccination in mice leading to reduced antigen-specific CD8+ T cell responses
Abstract
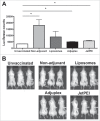
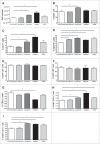
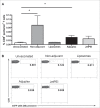
Adjuvants for DNA vaccination are designed to promote transformation of transgenes into target cells and increase inflammation in the site of injection, with resultant immune cell recruitment. Numerous studies indicated cationic liposomes as effective adjuvants for DNA vaccination due to their ability to promote in vivo transfection and innate immune system activation. Commercial reagents as Adjuplex and in vivo-JetPEI are also intended to facilitate DNA vaccination. Here, we evaluate the adjuvant properties of cationic liposomes, Adjuplex and in vivo-JetPEI compared to injection of DNA without adjuvant. In mice vaccinated with piggyBac pDNA vaccines, we assessed in vivo antigen expression, innate immune responses in draining lymph nodes, and antigen-specific T cell responses in spleens and blood. Surprisingly, vaccination with DNA in PBS emerged as the most efficient in promoting in vivo transfection and consequent antigen expression, while the addition of adjuvant reduced the amount of antigen expressed. On the other hand, we discovered higher numbers of innate immune cells and activated dendritic cells in the lymph nodes of mice injected with adjuvants than those vaccinated in PBS. The analysis of eGFP-specific immune responses revealed that all the different immunizations induced functional antigen-specific T cells in spleens, although only T cells generated by non-adjuvant vaccination and Adjuplex were identified in the blood of vaccinated mice. These results provide insight into the effects of these 3 adjuvants and may facilitate appropriate use off adjuvants by researchers using DNA vaccines in laboratory animals.
Keywords: Adjuplex; DNA vaccine; JetPEI; adjuvants; cell mediated immunity; innate immunity; liposomes; piggyBac; transposase.
Figures
Similar articles
-
A short hairpin RNA-based adjuvant targeting NF-κB repressor IκBα promotes migration of dermal dendritic cells to draining lymph nodes and antitumor CTL responses induced by DNA vaccination.Vaccine. 2017 Jul 24;35(33):4148-4154. doi: 10.1016/j.vaccine.2017.06.041. Epub 2017 Jun 27. Vaccine. 2017. PMID: 28666759
-
Imiquimod and resiquimod in a mouse model: adjuvants for DNA vaccination by particle-mediated immunotherapeutic delivery.Vaccine. 2004 Apr 16;22(13-14):1799-809. doi: 10.1016/j.vaccine.2003.09.052. Vaccine. 2004. PMID: 15068864
-
Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells.J Immunol. 2014 Aug 15;193(4):1920-30. doi: 10.4049/jimmunol.1400948. Epub 2014 Jul 14. J Immunol. 2014. PMID: 25024381
-
Skin delivery of a hybrid liposome/ISCOM vaccine implicates a role for adjuvants in rapid modulation of inflammatory cells involved in innate immunity before the enhancement of adaptive immune responses.Immunol Cell Biol. 1998 Jun;76(3):245-55. doi: 10.1046/j.1440-1711.1998.00742.x. Immunol Cell Biol. 1998. PMID: 9682968 Review.
-
Insight into the immunobiology of human skin and functional specialization of skin dendritic cell subsets to innovate intradermal vaccination design.Curr Top Microbiol Immunol. 2012;351:25-76. doi: 10.1007/82_2011_169. Curr Top Microbiol Immunol. 2012. PMID: 21833835 Review.
Cited by
-
Immunization with a multi-antigen targeted DNA vaccine eliminates chemoresistant pancreatic cancer by disrupting tumor-stromal cell crosstalk.J Transl Med. 2023 Oct 9;21(1):702. doi: 10.1186/s12967-023-04519-3. J Transl Med. 2023. PMID: 37814317 Free PMC article.
-
DNA-Loaded Cationic Liposomes Efficiently Function as a Vaccine against Malarial Proteins.Mol Ther Methods Clin Dev. 2017 Aug 23;7:1-10. doi: 10.1016/j.omtm.2017.08.004. eCollection 2017 Dec 15. Mol Ther Methods Clin Dev. 2017. PMID: 28879213 Free PMC article.
-
Harnessing Recent Advances in Synthetic DNA and Electroporation Technologies for Rapid Vaccine Development Against COVID-19 and Other Emerging Infectious Diseases.Front Med Technol. 2020 Oct 21;2:571030. doi: 10.3389/fmedt.2020.571030. eCollection 2020. Front Med Technol. 2020. PMID: 35047878 Free PMC article. Review.
-
8th vaccine renaissance: A creative nexus for vaccine developers.Hum Vaccin Immunother. 2015;11(9):2294-5. doi: 10.1080/21645515.2015.1069453. Epub 2015 Jul 9. Hum Vaccin Immunother. 2015. PMID: 26158225 Free PMC article. No abstract available.
References
-
- Lu S. Immunogenicity of DNA vaccines in humans: it takes two to tango. Hum Vaccin 2008; 4:449-52; PMID:18443427; http://dx.doi.org/10.4161/hv.4.6.6179 - DOI - PubMed
-
- Sasaki S, Takeshita F, Xin KQ, Ishii N, Okuda K. Adjuvant formulations and delivery systems for DNA vaccines. Methods 2003; 31:243-54; PMID:14511957; http://dx.doi.org/10.1016/S1046-2023(03)00140-3 - DOI - PubMed
-
- Bertino P, Urschitz J, Hoffmann FW, You BR, Rose AH, Park WH, Moisyadi S, Hoffmann PR. Vaccination with a piggyBac plasmid with transgene integration potential leads to sustained antigen expression and CD8(+) T cell responses. Vaccine 2014; 32:1670-7; PMID:24513010; http://dx.doi.org/10.1016/j.vaccine.2014.01.063 - DOI - PMC - PubMed
-
- O'Hagan DT, De Gregorio E. The path to a successful vaccine adjuvant–‘the long and winding road’. Drug Discov Today 2009; 14:541-51; PMID:19508916; http://dx.doi.org/10.1016/j.drudis.2009.02.009 - DOI - PubMed
-
- McCartney S, Vermi W, Gilfillan S, Cella M, Murphy TL, Schreiber RD, Murphy KM, Colonna M. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med 2009; 206:2967-76; PMID:19995959; http://dx.doi.org/10.1084/jem.20091181 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
