Role of Host Cell p32 in Herpes Simplex Virus 1 De-Envelopment during Viral Nuclear Egress
- PMID: 26085152
- PMCID: PMC4524097
- DOI: 10.1128/JVI.01220-15
Role of Host Cell p32 in Herpes Simplex Virus 1 De-Envelopment during Viral Nuclear Egress
Abstract
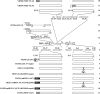
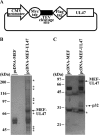
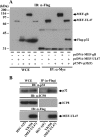
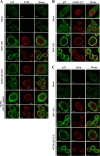
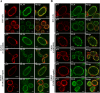
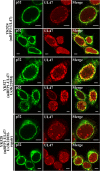
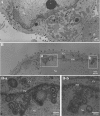
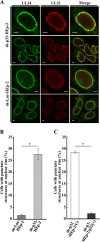
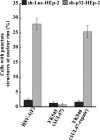
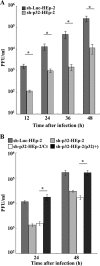
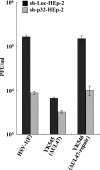
To clarify the function(s) of the herpes simplex virus 1 (HSV-1) major virion structural protein UL47 (also designated VP13/14), we screened cells overexpressing UL47 for UL47-binding cellular proteins. Tandem affinity purification of transiently expressed UL47 coupled with mass spectrometry-based proteomics technology and subsequent analyses showed that UL47 interacted with cell protein p32 in HSV-1-infected cells. Unlike in mock-infected cells, p32 accumulated at the nuclear rim in HSV-1-infected cells, and this p32 recruitment to the nuclear rim required UL47. p32 formed a complex(es) with HSV-1 proteins UL31, UL34, Us3, UL47, and/or ICP22 in HSV-1-infected cells. All these HSV-1 proteins were previously reported to be important for HSV-1 nuclear egress, in which nucleocapsids bud through the inner nuclear membrane (primary envelopment) and the enveloped nucleocapsids then fuse with the outer nuclear membrane (de-envelopment). Like viral proteins UL31, UL34, Us3, and UL47, p32 was detected in primary enveloped virions. p32 knockdown reduced viral replication and induced membranous invaginations adjacent to the nuclear rim containing primary enveloped virions and aberrant localization of UL31 and UL34 in punctate structures at the nuclear rim. These effects of p32 knockdown were reduced in the absence of UL47. Therefore, the effects of p32 knockdown in HSV-1 nuclear egress were similar to those of the previously reported mutation(s) in HSV-1 regulatory proteins for HSV-1 de-envelopment during viral nuclear egress. Collectively, these results suggested that p32 regulated HSV-1 de-envelopment and replication in a UL47-dependent manner. IMPORTANCE In this study, we have obtained data suggesting that (i) the HSV-1 major virion structural protein UL47 interacted with host cell protein p32 and mediated the recruitment of p32 to the nuclear rim in HSV-1-infected cells; (ii) p32 was a component of the HSV-1 nuclear egress complex (NEC), whose core components were UL31 and UL34; and (iii) p32 regulated HSV-1 de-envelopment during viral nuclear egress. It has been reported that p32 was a component of human cytomegalovirus NEC and was required for efficient disintegration of nuclear lamina, which has been thought to facilitate HSV-1 primary envelopment during viral nuclear egress. Thus, p32 appeared to be a core component of herpesvirus NECs, like UL31 and UL34 homologs in other herpesviruses, and to play multiple roles in herpesvirus nuclear egress.
Figures




Similar articles
-
Herpes simplex virus 1 UL47 interacts with viral nuclear egress factors UL31, UL34, and Us3 and regulates viral nuclear egress.J Virol. 2014 May;88(9):4657-67. doi: 10.1128/JVI.00137-14. Epub 2014 Feb 12. J Virol. 2014. PMID: 24522907 Free PMC article.
-
Role of herpes simplex virus 1 immediate early protein ICP22 in viral nuclear egress.J Virol. 2014 Jul;88(13):7445-54. doi: 10.1128/JVI.01057-14. Epub 2014 Apr 16. J Virol. 2014. PMID: 24741100 Free PMC article.
-
Identification of the Capsid Binding Site in the Herpes Simplex Virus 1 Nuclear Egress Complex and Its Role in Viral Primary Envelopment and Replication.J Virol. 2019 Oct 15;93(21):e01290-19. doi: 10.1128/JVI.01290-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31391274 Free PMC article.
-
Herpesvirus Nuclear Egress across the Outer Nuclear Membrane.Viruses. 2021 Nov 24;13(12):2356. doi: 10.3390/v13122356. Viruses. 2021. PMID: 34960625 Free PMC article. Review.
-
Host and Viral Factors Involved in Nuclear Egress of Herpes Simplex Virus 1.Viruses. 2021 Apr 25;13(5):754. doi: 10.3390/v13050754. Viruses. 2021. PMID: 33923040 Free PMC article. Review.
Cited by
-
Roles of the Phosphorylation of Herpes Simplex Virus 1 UL51 at a Specific Site in Viral Replication and Pathogenicity.J Virol. 2018 Aug 29;92(18):e01035-18. doi: 10.1128/JVI.01035-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29976672 Free PMC article.
-
Herpes Simplex Virus: The Hostile Guest That Takes Over Your Home.Front Microbiol. 2020 May 7;11:733. doi: 10.3389/fmicb.2020.00733. eCollection 2020. Front Microbiol. 2020. PMID: 32457704 Free PMC article. Review.
-
ESCRT-III mediates budding across the inner nuclear membrane and regulates its integrity.Nat Commun. 2018 Aug 23;9(1):3379. doi: 10.1038/s41467-018-05889-9. Nat Commun. 2018. PMID: 30139939 Free PMC article.
-
'Shared-Hook' and 'Changed-Hook' Binding Activities of Herpesviral Core Nuclear Egress Complexes Identified by Random Mutagenesis.Cells. 2022 Dec 13;11(24):4030. doi: 10.3390/cells11244030. Cells. 2022. PMID: 36552794 Free PMC article.
-
The Oligomeric Assemblies of Cytomegalovirus Core Nuclear Egress Proteins Are Associated with Host Kinases and Show Sensitivity to Antiviral Kinase Inhibitors.Viruses. 2022 May 11;14(5):1021. doi: 10.3390/v14051021. Viruses. 2022. PMID: 35632762 Free PMC article.
References
-
- Reynolds AE, Ryckman BJ, Baines JD, Zhou Y, Liang L, Roller RJ. 2001. U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J Virol 75:8803–8817. doi:10.1128/JVI.75.18.8803-8817.2001. - DOI - PMC - PubMed
-
- Roizman B, Knipe DM, Whitley RJ. 2013. Herpes simplex viruses, p 1823–1897. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

