Biology of Zika Virus Infection in Human Skin Cells
- PMID: 26085147
- PMCID: PMC4524089
- DOI: 10.1128/JVI.00354-15
Biology of Zika Virus Infection in Human Skin Cells
Abstract
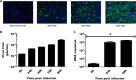
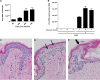
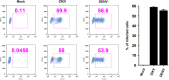
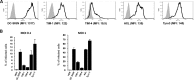
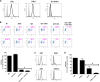
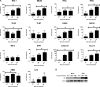
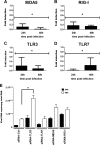
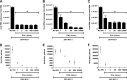
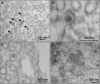
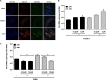
Zika virus (ZIKV) is an emerging arbovirus of the Flaviviridae family, which includes dengue, West Nile, yellow fever, and Japanese encephalitis viruses, that causes a mosquito-borne disease transmitted by the Aedes genus, with recent outbreaks in the South Pacific. Here we examine the importance of human skin in the entry of ZIKV and its contribution to the induction of antiviral immune responses. We show that human dermal fibroblasts, epidermal keratinocytes, and immature dendritic cells are permissive to the most recent ZIKV isolate, responsible for the epidemic in French Polynesia. Several entry and/or adhesion factors, including DC-SIGN, AXL, Tyro3, and, to a lesser extent, TIM-1, permitted ZIKV entry, with a major role for the TAM receptor AXL. The ZIKV permissiveness of human skin fibroblasts was confirmed by the use of a neutralizing antibody and specific RNA silencing. ZIKV induced the transcription of Toll-like receptor 3 (TLR3), RIG-I, and MDA5, as well as several interferon-stimulated genes, including OAS2, ISG15, and MX1, characterized by strongly enhanced beta interferon gene expression. ZIKV was found to be sensitive to the antiviral effects of both type I and type II interferons. Finally, infection of skin fibroblasts resulted in the formation of autophagosomes, whose presence was associated with enhanced viral replication, as shown by the use of Torin 1, a chemical inducer of autophagy, and the specific autophagy inhibitor 3-methyladenine. The results presented herein permit us to gain further insight into the biology of ZIKV and to devise strategies aiming to interfere with the pathology caused by this emerging flavivirus.
Importance: Zika virus (ZIKV) is an arbovirus belonging to the Flaviviridae family. Vector-mediated transmission of ZIKV is initiated when a blood-feeding female Aedes mosquito injects the virus into the skin of its mammalian host, followed by infection of permissive cells via specific receptors. Indeed, skin immune cells, including dermal fibroblasts, epidermal keratinocytes, and immature dendritic cells, were all found to be permissive to ZIKV infection. The results also show a major role for the phosphatidylserine receptor AXL as a ZIKV entry receptor and for cellular autophagy in enhancing ZIKV replication in permissive cells. ZIKV replication leads to activation of an antiviral innate immune response and the production of type I interferons in infected cells. Taken together, these results provide the first general insights into the interaction between ZIKV and its mammalian host.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
STAT5: a Target of Antagonism by Neurotropic Flaviviruses.J Virol. 2019 Nov 13;93(23):e00665-19. doi: 10.1128/JVI.00665-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31534033 Free PMC article.
-
Dengue virus replication in infected human keratinocytes leads to activation of antiviral innate immune responses.Infect Genet Evol. 2011 Oct;11(7):1664-73. doi: 10.1016/j.meegid.2011.06.009. Epub 2011 Jun 21. Infect Genet Evol. 2011. PMID: 21722754
-
Characterization of zika virus infection of human fetal cardiac mesenchymal stromal cells.PLoS One. 2020 Sep 17;15(9):e0239238. doi: 10.1371/journal.pone.0239238. eCollection 2020. PLoS One. 2020. PMID: 32941515 Free PMC article.
-
Subverting the mechanisms of cell death: flavivirus manipulation of host cell responses to infection.Biochem Soc Trans. 2018 Jun 19;46(3):609-617. doi: 10.1042/BST20170399. Epub 2018 Apr 20. Biochem Soc Trans. 2018. PMID: 29678952 Review.
-
Pathogenesis and Manifestations of Zika Virus-Associated Ocular Diseases.Trop Med Infect Dis. 2022 Jun 15;7(6):106. doi: 10.3390/tropicalmed7060106. Trop Med Infect Dis. 2022. PMID: 35736984 Free PMC article. Review.
Cited by
-
Taking AIM at Influenza: The Role of the AIM2 Inflammasome.Viruses. 2024 Sep 27;16(10):1535. doi: 10.3390/v16101535. Viruses. 2024. PMID: 39459869 Free PMC article. Review.
-
Zika Virus Infects Human Placental Macrophages.Cell Host Microbe. 2016 Jul 13;20(1):83-90. doi: 10.1016/j.chom.2016.05.015. Epub 2016 May 27. Cell Host Microbe. 2016. PMID: 27247001 Free PMC article.
-
A Mouse Model of Zika Virus Pathogenesis.Cell Host Microbe. 2016 May 11;19(5):720-30. doi: 10.1016/j.chom.2016.03.010. Epub 2016 Apr 5. Cell Host Microbe. 2016. PMID: 27066744 Free PMC article.
-
Antagonism of interferon signaling by fibroblast growth factors promotes viral replication.EMBO Mol Med. 2020 Sep 7;12(9):e11793. doi: 10.15252/emmm.201911793. Epub 2020 Jul 27. EMBO Mol Med. 2020. PMID: 32720440 Free PMC article.
-
Insights into mosquito-borne arbovirus receptors.Cell Insight. 2024 Aug 24;3(6):100196. doi: 10.1016/j.cellin.2024.100196. eCollection 2024 Dec. Cell Insight. 2024. PMID: 39391003 Free PMC article. Review.
References
-
- Moore DL, Causey OR, Carey DE, Reddy S, Cooke AR, Akinkugbe FM, David-West TS, Kemp GE. 1975. Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann Trop Med Parasitol 69:49–64. - PubMed
-
- Smithburn KC. 1954. Neutralizing antibodies against arthropod-borne viruses in the sera of long-time residents of Malaya and Borneo. Am J Hyg 59:157–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

