Spatiotemporal control of a novel synaptic organizer molecule
- PMID: 26083757
- PMCID: PMC9134992
- DOI: 10.1038/nature14545
Spatiotemporal control of a novel synaptic organizer molecule
Abstract
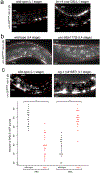
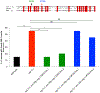
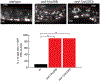
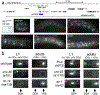
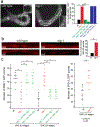
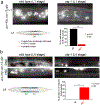
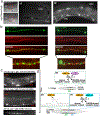
Synapse formation is a process tightly controlled in space and time. How gene regulatory mechanisms specify spatial and temporal aspects of synapse formation is not well understood. In the nematode Caenorhabditis elegans, two subtypes of the D-type inhibitory motor neuron (MN) classes, the dorsal D (DD) and ventral D (VD) neurons, extend axons along both the dorsal and ventral nerve cords. The embryonically generated DD motor neurons initially innervate ventral muscles in the first (L1) larval stage and receive their synaptic input from cholinergic motor neurons in the dorsal cord. They rewire by the end of the L1 moult to innervate dorsal muscles and to be innervated by newly formed ventral cholinergic motor neurons. VD motor neurons develop after the L1 moult; they take over the innervation of ventral muscles and receive their synaptic input from dorsal cholinergic motor neurons. We show here that the spatiotemporal control of synaptic wiring of the D-type neurons is controlled by an intersectional transcriptional strategy in which the UNC-30 Pitx-type homeodomain transcription factor acts together, in embryonic and early larval stages, with the temporally controlled LIN-14 transcription factor to prevent premature synapse rewiring of the DD motor neurons and, together with the UNC-55 nuclear hormone receptor, to prevent aberrant VD synaptic wiring in later larval and adult stages. A key effector of this intersectional transcription factor combination is a novel synaptic organizer molecule, the single immunoglobulin domain protein OIG-1. OIG-1 is perisynaptically localized along the synaptic outputs of the D-type motor neurons in a temporally controlled manner and is required for appropriate selection of both pre- and post-synaptic partners.
Figures
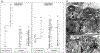






Comment in
-
Neurobiology: Inversion in the worm.Nature. 2015 Jul 2;523(7558):44-5. doi: 10.1038/523044a. Nature. 2015. PMID: 26135446 Free PMC article.
Similar articles
-
Transcriptional Control of Synaptic Remodeling through Regulated Expression of an Immunoglobulin Superfamily Protein.Curr Biol. 2015 Oct 5;25(19):2541-8. doi: 10.1016/j.cub.2015.08.022. Epub 2015 Sep 17. Curr Biol. 2015. PMID: 26387713 Free PMC article.
-
Convergent genetic programs regulate similarities and differences between related motor neuron classes in Caenorhabditis elegans.Dev Biol. 2005 Apr 15;280(2):494-503. doi: 10.1016/j.ydbio.2005.01.032. Dev Biol. 2005. PMID: 15882588
-
UNC-55, an orphan nuclear hormone receptor, orchestrates synaptic specificity among two classes of motor neurons in Caenorhabditis elegans.J Neurosci. 1998 Dec 15;18(24):10438-44. doi: 10.1523/JNEUROSCI.18-24-10438.1998. J Neurosci. 1998. PMID: 9852581 Free PMC article.
-
Convergent Transcriptional Programs Regulate cAMP Levels in C. elegans GABAergic Motor Neurons.Dev Cell. 2017 Oct 23;43(2):212-226.e7. doi: 10.1016/j.devcel.2017.09.013. Epub 2017 Oct 12. Dev Cell. 2017. PMID: 29033363
-
Intrinsic and extrinsic mechanisms of synapse formation and specificity in C. elegans.Cell Mol Life Sci. 2019 Jul;76(14):2719-2738. doi: 10.1007/s00018-019-03109-1. Epub 2019 Apr 29. Cell Mol Life Sci. 2019. PMID: 31037336 Free PMC article. Review.
Cited by
-
The physiological function of long-noncoding RNAs.Noncoding RNA Res. 2020 Dec;5(4):178-184. doi: 10.1016/j.ncrna.2020.09.003. Epub 2020 Sep 17. Noncoding RNA Res. 2020. PMID: 32959025 Free PMC article. Review.
-
Timing of neuronal plasticity in development and aging.Wiley Interdiscip Rev Dev Biol. 2018 Mar;7(2):10.1002/wdev.305. doi: 10.1002/wdev.305. Epub 2017 Nov 15. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 29139210 Free PMC article. Review.
-
The Hox Gene egl-5 Acts as a Terminal Selector for VD13 Development via Wnt Signaling.J Dev Biol. 2020 Mar 3;8(1):5. doi: 10.3390/jdb8010005. J Dev Biol. 2020. PMID: 32138237 Free PMC article.
-
A cellular and regulatory map of the GABAergic nervous system of C. elegans.Elife. 2016 Oct 14;5:e17686. doi: 10.7554/eLife.17686. Elife. 2016. PMID: 27740909 Free PMC article.
-
The Prop1-like homeobox gene unc-42 specifies the identity of synaptically connected neurons.Elife. 2021 Jun 24;10:e64903. doi: 10.7554/eLife.64903. Elife. 2021. PMID: 34165428 Free PMC article.
References
-
- White JG, Albertson DG & Anness MA Connectivity changes in a class of motoneurone during the development of a nematode. Nature 271, 764–6 (1978). - PubMed
-
- Jin Y, Hoskins R & Horvitz HR Control of type-D GABAergic neuron differentiation by C. elegans UNC-30 homeodomain protein. Nature 372, 780–3 (1994). - PubMed
-
- Hallam SJ & Jin Y lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature 395, 78–82 (1998). - PubMed
-
- Ruvkun G & Giusto J The Caenorhabditis elegans heterochronic gene lin-14 encodes a nuclear protein that forms a temporal developmental switch. Nature 338, 313–9 (1989). - PubMed
REFERENCES FOR ONLINE MATERIAL
-
- Gendrel M, Rapti G, Richmond JE & Bessereau JL A secreted complement-control-related protein ensures acetylcholine receptor clustering. Nature 461, 992–6 (2009). - PubMed
-
- Duerr JS, Han HP, Fields SD & Rand JB Identification of major classes of cholinergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol 506, 398–408 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

