Rimonabant Improves Oxidative/Nitrosative Stress in Mice with Nonalcoholic Fatty Liver Disease
- PMID: 26078820
- PMCID: PMC4442287
- DOI: 10.1155/2015/842108
Rimonabant Improves Oxidative/Nitrosative Stress in Mice with Nonalcoholic Fatty Liver Disease
Abstract
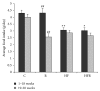
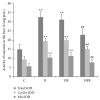
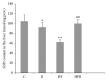
The present study deals with the effects of rimonabant on oxidative/nitrosative stress in high diet- (HFD-) induced experimental nonalcoholic fatty liver disease (NAFLD). Male mice C57BL/6 were divided into the following groups: control group fed with control diet for 20 weeks (C; n = 6); group fed with HFD for 20 weeks (HF; n = 6); group fed with standard diet and treated with rimonabant after 18 weeks (R; n = 9); group fed with HFD and treated with rimonabant after 18 weeks (HFR; n = 10). Daily dose of rimonabant (10 mg/kg) was administered to HFR and R group by oral gavage for two weeks. Treatment induced a decrease in hepatic malondialdehyde concentration in HFR group compared to HF group (P < 0.01). The concentration of nitrites + nitrates in liver was decreased in HFR group compared to HF group (P < 0.01). Liver content of reduced glutathione was higher in HFR group compared to HF group (P < 0.01). Total liver superoxide dismutase activity in HFR group was decreased in comparison with HF group (P < 0.01). It was found that rimonabant may influence hepatic iron, zinc, copper, and manganese status. Our study indicates potential usefulness of cannabinoid receptor type 1 blockade in the treatment of HFD-induced NAFLD.
Figures


Similar articles
-
The effect of cannabinoid receptor 1 blockade on hepatic free fatty acid profile in mice with nonalcoholic fatty liver disease.Chem Phys Lipids. 2017 Apr;204:85-93. doi: 10.1016/j.chemphyslip.2017.03.009. Epub 2017 Mar 29. Chem Phys Lipids. 2017. PMID: 28363784
-
The effect of cannabinoid receptor 1 blockade on adipokine and proinflammatory cytokine concentration in adipose and hepatic tissue in mice with nonalcoholic fatty liver disease.Can J Physiol Pharmacol. 2019 Feb;97(2):120-129. doi: 10.1139/cjpp-2018-0607. Epub 2019 Jan 23. Can J Physiol Pharmacol. 2019. PMID: 30673308
-
Dynamics of oxidative/nitrosative stress in mice with methionine-choline-deficient diet-induced nonalcoholic fatty liver disease.Hum Exp Toxicol. 2014 Jul;33(7):701-9. doi: 10.1177/0960327113506723. Epub 2013 Oct 15. Hum Exp Toxicol. 2014. PMID: 24130212
-
Protective effect of rimonabant, a canabinoid receptor 1 antagonist, on nonalcoholic fatty liver disease in a rat model through modulation of the hepatic expression of activin A and follistatin.Can J Physiol Pharmacol. 2017 Dec;95(12):1433-1441. doi: 10.1139/cjpp-2017-0070. Epub 2017 Jul 31. Can J Physiol Pharmacol. 2017. PMID: 28759733
-
Weight loss enhances hepatic antioxidant status in a NAFLD model induced by high-fat diet.Appl Physiol Nutr Metab. 2018 Jan;43(1):23-29. doi: 10.1139/apnm-2017-0317. Epub 2017 Aug 23. Appl Physiol Nutr Metab. 2018. PMID: 28834687
Cited by
-
Fish oil supplementation during pregnancy decreases liver endocannabinoid system and lipogenic markers in newborn rats exposed to maternal high-fat diet.Eur J Nutr. 2024 Aug;63(5):1565-1579. doi: 10.1007/s00394-024-03422-x. Epub 2024 May 10. Eur J Nutr. 2024. PMID: 38727803
-
Therapeutic Approaches for Nonalcoholic Fatty Liver Disease: Established Targets and Drugs.Diabetes Metab Syndr Obes. 2023 Jun 21;16:1809-1819. doi: 10.2147/DMSO.S411400. eCollection 2023. Diabetes Metab Syndr Obes. 2023. PMID: 37366486 Free PMC article. Review.
-
Maternal high-fat diet consumption induces sex-dependent alterations of the endocannabinoid system and redox homeostasis in liver of adult rat offspring.Sci Rep. 2018 Oct 3;8(1):14751. doi: 10.1038/s41598-018-32906-0. Sci Rep. 2018. PMID: 30282988 Free PMC article.
-
Adipose-derived extracellular vesicles - a novel cross-talk mechanism in insulin resistance, non-alcoholic fatty liver disease, and polycystic ovary syndrome.Endocrine. 2024 Jul;85(1):18-34. doi: 10.1007/s12020-024-03702-w. Epub 2024 Jan 29. Endocrine. 2024. PMID: 38285412 Review.
-
Potential Implications of Rimonabant on Age-Related Oxidative Stress and Inflammation.Antioxidants (Basel). 2022 Jan 14;11(1):162. doi: 10.3390/antiox11010162. Antioxidants (Basel). 2022. PMID: 35052666 Free PMC article.
References
-
- Fraulob J. C., Ogg-Diamantino R., Fernandes-Santos C., Aguila M. B., Mandarim-de-Lacerda C. A. A mouse model of metabolic syndrome: insulin resistance, fatty liver and Non-Alcoholic Fatty Pancreas Disease (NAFPD) in C57BL/6 mice fed a high fat diet. Journal of Clinical Biochemistry and Nutrition. 2010;46(3):212–223. doi: 10.3164/jcbn.09-83. - DOI - PMC - PubMed
-
- Aubert J., Begriche K., Knockaert L., Robin M. A., Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clinics and Research in Hepatology and Gastroenterology. 2011;35(10):630–637. doi: 10.1016/j.clinre.2011.04.015. - DOI - PubMed
-
- Seki S., Kitada T., Sakaguchi H. Clinicopathological significance of oxidative cellular damage in non-alcoholic fatty liver diseases. Hepatology Research. 2005;33(2):132–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

