Robust Distal Tip Cell Pathfinding in the Face of Temperature Stress Is Ensured by Two Conserved microRNAS in Caenorhabditis elegans
- PMID: 26078280
- PMCID: PMC4574240
- DOI: 10.1534/genetics.115.179184
Robust Distal Tip Cell Pathfinding in the Face of Temperature Stress Is Ensured by Two Conserved microRNAS in Caenorhabditis elegans
Abstract
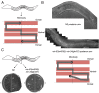
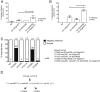
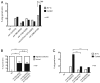
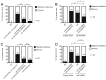
Biological robustness, the ability of an organism to maintain a steady-state output as genetic or environmental inputs change, is critical for proper development. MicroRNAs have been implicated in biological robustness mechanisms through their post-transcriptional regulation of genes and gene networks. Previous research has illustrated examples of microRNAs promoting robustness as part of feedback loops and genetic switches and by buffering noisy gene expression resulting from environmental and/or internal changes. Here we show that the evolutionarily conserved microRNAs mir-34 and mir-83 (homolog of mammalian mir-29) contribute to the robust migration pattern of the distal tip cells in Caenorhabditis elegans by specifically protecting against stress from temperature changes. Furthermore, our results indicate that mir-34 and mir-83 may modulate the integrin signaling involved in distal tip cell migration by potentially targeting the GTPase cdc-42 and the beta-integrin pat-3. Our findings suggest a role for mir-34 and mir-83 in integrin-controlled cell migrations that may be conserved through higher organisms. They also provide yet another example of microRNA-based developmental robustness in response to a specific environmental stress, rapid temperature fluctuations.
Keywords: distal tip cell migrations; mir-29; mir-34; mir-83; robustness.
Copyright © 2015 by the Genetics Society of America.
Figures

Similar articles
-
MicroRNA mir-34 provides robustness to environmental stress response via the DAF-16 network in C. elegans.Sci Rep. 2016 Dec 1;6:36766. doi: 10.1038/srep36766. Sci Rep. 2016. PMID: 27905558 Free PMC article.
-
miR-124/ATF-6, a novel lifespan extension pathway of Astragalus polysaccharide in Caenorhabditis elegans.J Cell Biochem. 2015 Feb;116(2):242-51. doi: 10.1002/jcb.24961. J Cell Biochem. 2015. PMID: 25186652
-
miR-71 mediates age-dependent opposing contributions of the stress-activated kinase KGB-1 in Caenorhabditis elegans.Genetics. 2021 Jun 24;218(2):iyab049. doi: 10.1093/genetics/iyab049. Genetics. 2021. PMID: 33755114 Free PMC article.
-
Roles of microRNAs in the Caenorhabditis elegans nervous system.J Genet Genomics. 2013 Sep 20;40(9):445-52. doi: 10.1016/j.jgg.2013.07.002. Epub 2013 Aug 7. J Genet Genomics. 2013. PMID: 24053946 Review.
-
Uncovering new functions for microRNAs in Caenorhabditis elegans.Curr Biol. 2011 Sep 13;21(17):R668-71. doi: 10.1016/j.cub.2011.07.027. Curr Biol. 2011. PMID: 21920301 Free PMC article. Review.
Cited by
-
Remodeling of the Caenorhabditis elegans non-coding RNA transcriptome by heat shock.Nucleic Acids Res. 2019 Oct 10;47(18):9829-9841. doi: 10.1093/nar/gkz693. Nucleic Acids Res. 2019. PMID: 31396626 Free PMC article.
-
TDP-1 and FUST-1 co-inhibit exon inclusion and control fertility together with transcriptional regulation.Nucleic Acids Res. 2023 Oct 13;51(18):9610-9628. doi: 10.1093/nar/gkad665. Nucleic Acids Res. 2023. PMID: 37587694 Free PMC article.
-
A secreted microRNA disrupts autophagy in distinct tissues of Caenorhabditis elegans upon ageing.Nat Commun. 2019 Oct 23;10(1):4827. doi: 10.1038/s41467-019-12821-2. Nat Commun. 2019. PMID: 31645592 Free PMC article.
-
Mathematics of microRNAs: stabilizing gene regulatory networks.Natl Sci Rev. 2019 Nov;6(6):1189-1190. doi: 10.1093/nsr/nwz112. Epub 2019 Aug 13. Natl Sci Rev. 2019. PMID: 34691997 Free PMC article. No abstract available.
-
Integrin restriction by miR-34 protects germline progenitors from cell death during aging.Aging Cell. 2024 Jun;23(6):e14131. doi: 10.1111/acel.14131. Epub 2024 Mar 7. Aging Cell. 2024. PMID: 38450871 Free PMC article.
References
-
- Ambros V., 2004. The functions of animal microRNAs. Nature 431: 350–355. - PubMed
-
- Ambros V., Lee R. C., Lavanway A., Williams P. T., Jewell D., 2003. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr. Biol. 13: 807–818. - PubMed
-
- Aravin A. A., Lagos-Quintana M., Yalcin A., Zavolan M., Marks D., et al. , 2003. The small RNA profile during Drosophila melanogaster development. Dev. Cell 5: 337–350. - PubMed
-
- Bartel D. P., 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

