Insights into primary immune deficiency from quantitative microscopy
- PMID: 26078103
- PMCID: PMC4641025
- DOI: 10.1016/j.jaci.2015.03.049
Insights into primary immune deficiency from quantitative microscopy
Abstract
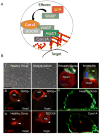
Recent advances in genomics-based technology have resulted in an increase in our understanding of the molecular basis of many primary immune deficiencies. Along with this increased knowledge comes an increased responsibility to understand the underlying mechanism of disease, and thus increasingly sophisticated technologies are being used to investigate the cell biology of human immune deficiencies. One such technology, which has itself undergone a recent explosion in innovation, is that of high-resolution microscopy and image analysis. These advances complement innovative studies that have previously shed light on critical cell biological processes that are perturbed by single-gene mutations in primary immune deficiency. Here we highlight advances made specifically in the following cell biological processes: (1) cytoskeletal-related processes; (2) cell signaling; (3) intercellular trafficking; and (4) cellular host defense.
Keywords: Primary immune deficiency; cell biology; cytoskeleton; host defense; microscopy.
Copyright © 2015 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Proteomic approaches to understanding the role of the cytoskeleton in host-defense mechanisms.Expert Rev Proteomics. 2011 Feb;8(1):117-26. doi: 10.1586/epr.10.91. Expert Rev Proteomics. 2011. PMID: 21329431 Free PMC article. Review.
-
Visualizing a role for the actin cytoskeleton in the regulation of B-cell activation.Immunol Rev. 2010 Sep;237(1):191-204. doi: 10.1111/j.1600-065X.2010.00943.x. Immunol Rev. 2010. PMID: 20727037 Review.
-
Immune pathology associated with altered actin cytoskeleton regulation.Autoimmunity. 2010 Feb;43(1):64-75. doi: 10.3109/08916930903374634. Autoimmunity. 2010. PMID: 20001423 Free PMC article. Review.
-
Understanding Cytoskeletal Dynamics During the Plant Immune Response.Annu Rev Phytopathol. 2018 Aug 25;56:513-533. doi: 10.1146/annurev-phyto-080516-035632. Epub 2018 Jul 5. Annu Rev Phytopathol. 2018. PMID: 29975609 Review.
-
The intersection of immune deficiency and autoimmunity.Curr Opin Rheumatol. 2014 Sep;26(5):570-8. doi: 10.1097/BOR.0000000000000091. Curr Opin Rheumatol. 2014. PMID: 25014038 Review.
Cited by
-
Advances in clinical immunology in 2015.J Allergy Clin Immunol. 2016 Dec;138(6):1531-1540. doi: 10.1016/j.jaci.2016.10.005. J Allergy Clin Immunol. 2016. PMID: 27931534 Free PMC article. Review.
-
Emerging insights into human health and NK cell biology from the study of NK cell deficiencies.Immunol Rev. 2019 Jan;287(1):202-225. doi: 10.1111/imr.12725. Immunol Rev. 2019. PMID: 30565241 Free PMC article. Review.
-
Actin Dynamics at the T Cell Synapse as Revealed by Immune-Related Actinopathies.Front Cell Dev Biol. 2021 Jun 24;9:665519. doi: 10.3389/fcell.2021.665519. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34249918 Free PMC article. Review.
References
-
- Massaad MJ, Ramesh N, Geha RS. Wiskott-Aldrich syndrome: a comprehensive review. Ann N Y Acad Sci. 2013;1285:26–43. - PubMed
-
- Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78(4):635–44. - PubMed
-
- Remold-O'Donnell E, Rosen FS, Kenney DM. Defects in Wiskott-Aldrich syndrome blood cells. Blood. 1996;87(7):2621–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

