DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice
- PMID: 26077864
- PMCID: PMC4490365
- DOI: 10.1038/ncomms8415
DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice
Abstract
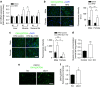
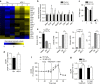
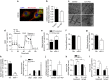
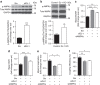
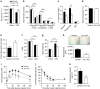
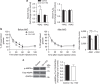
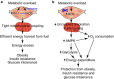
Reactive oxygen species (ROS) have been linked to a wide variety of pathologies, including obesity and diabetes, but ROS also act as endogenous signalling molecules, regulating numerous biological processes. DJ-1 is one of the most evolutionarily conserved proteins across species, and mutations in DJ-1 have been linked to some cases of Parkinson's disease. Here we show that DJ-1 maintains cellular metabolic homeostasis via modulating ROS levels in murine skeletal muscles, revealing a role of DJ-1 in maintaining efficient fuel utilization. We demonstrate that, in the absence of DJ-1, ROS uncouple mitochondrial respiration and activate AMP-activated protein kinase, which triggers Warburg-like metabolic reprogramming in muscle cells. Accordingly, DJ-1 knockout mice exhibit higher energy expenditure and are protected from obesity, insulin resistance and diabetes in the setting of fuel surplus. Our data suggest that promoting mitochondrial uncoupling may be a potential strategy for the treatment of obesity-associated metabolic disorders.
Figures
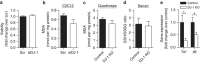
Similar articles
-
ROS-dependent regulation of Parkin and DJ-1 localization during oxidative stress in neurons.Hum Mol Genet. 2012 Nov 15;21(22):4888-903. doi: 10.1093/hmg/dds325. Epub 2012 Aug 7. Hum Mol Genet. 2012. PMID: 22872702
-
Loss of DJ-1 does not affect mitochondrial respiration but increases ROS production and mitochondrial permeability transition pore opening.PLoS One. 2012;7(7):e40501. doi: 10.1371/journal.pone.0040501. Epub 2012 Jul 9. PLoS One. 2012. PMID: 22792356 Free PMC article.
-
The development of diet-induced obesity and associated metabolic impairments in Dj-1 deficient mice.J Nutr Biochem. 2015 Jan;26(1):75-81. doi: 10.1016/j.jnutbio.2014.09.002. Epub 2014 Oct 2. J Nutr Biochem. 2015. PMID: 25448609
-
The Role of the Antioxidant Protein DJ-1 in Type 2 Diabetes Mellitus.Adv Exp Med Biol. 2017;1037:173-186. doi: 10.1007/978-981-10-6583-5_11. Adv Exp Med Biol. 2017. PMID: 29147909 Review.
-
Epigallocatechin Gallate Modulates Muscle Homeostasis in Type 2 Diabetes and Obesity by Targeting Energetic and Redox Pathways: A Narrative Review.Int J Mol Sci. 2019 Jan 27;20(3):532. doi: 10.3390/ijms20030532. Int J Mol Sci. 2019. PMID: 30691224 Free PMC article. Review.
Cited by
-
CaMKIV regulates mitochondrial dynamics during sepsis.Cell Calcium. 2020 Dec;92:102286. doi: 10.1016/j.ceca.2020.102286. Epub 2020 Sep 5. Cell Calcium. 2020. PMID: 32932146 Free PMC article.
-
Network analysis reveals common host protein/s modulating pathogenesis of neurotropic viruses.Sci Rep. 2016 Sep 1;6:32593. doi: 10.1038/srep32593. Sci Rep. 2016. PMID: 27581498 Free PMC article.
-
Is Mitochondrial Dysfunction a Common Root of Noncommunicable Chronic Diseases?Endocr Rev. 2020 Jun 1;41(3):bnaa005. doi: 10.1210/endrev/bnaa005. Endocr Rev. 2020. PMID: 32179913 Free PMC article. Review.
-
Janus Kinase 2 (JAK2) Dissociates Hepatosteatosis from Hepatocellular Carcinoma in Mice.J Biol Chem. 2017 Mar 3;292(9):3789-3799. doi: 10.1074/jbc.M116.752519. Epub 2017 Jan 18. J Biol Chem. 2017. PMID: 28100771 Free PMC article.
-
The mitochondrial metabolic function of DJ-1 is modulated by 14-3-3β.FASEB J. 2019 Aug;33(8):8925-8934. doi: 10.1096/fj.201802754R. Epub 2019 Apr 29. FASEB J. 2019. PMID: 31034784 Free PMC article.
References
-
- Castresana J. & Saraste M. Evolution of energetic metabolism: the respiration-early hypothesis. Trends Biochem. Sci. 20, 443–448 (1995). - PubMed
-
- Zimmet P. & Thomas C. R. Genotype, obesity and cardiovascular disease--has technical and social advancement outstripped evolution? J. Intern. Med. 254, 114–125 (2003). - PubMed
-
- Rhee S. G. Cell signaling. H2O2, a necessay evil for cell signaling. Science 312, 1882–1883 (2006). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

