A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules
- PMID: 26077718
- PMCID: PMC4493418
- DOI: 10.1084/jem.20142290
A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules
Abstract
The central nervous system (CNS) is considered an organ devoid of lymphatic vasculature. Yet, part of the cerebrospinal fluid (CSF) drains into the cervical lymph nodes (LNs). The mechanism of CSF entry into the LNs has been unclear. Here we report the surprising finding of a lymphatic vessel network in the dura mater of the mouse brain. We show that dural lymphatic vessels absorb CSF from the adjacent subarachnoid space and brain interstitial fluid (ISF) via the glymphatic system. Dural lymphatic vessels transport fluid into deep cervical LNs (dcLNs) via foramina at the base of the skull. In a transgenic mouse model expressing a VEGF-C/D trap and displaying complete aplasia of the dural lymphatic vessels, macromolecule clearance from the brain was attenuated and transport from the subarachnoid space into dcLNs was abrogated. Surprisingly, brain ISF pressure and water content were unaffected. Overall, these findings indicate that the mechanism of CSF flow into the dcLNs is directly via an adjacent dural lymphatic network, which may be important for the clearance of macromolecules from the brain. Importantly, these results call for a reexamination of the role of the lymphatic system in CNS physiology and disease.
© 2015 Aspelund et al.
Figures
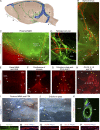
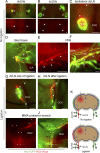
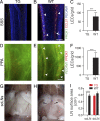
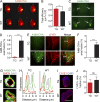
Comment in
-
Implications of the discovery of brain lymphatic pathways.Lancet Neurol. 2015 Oct;14(10):977-9. doi: 10.1016/S1474-4422(15)00221-5. Lancet Neurol. 2015. PMID: 26376966 Free PMC article. No abstract available.
-
Lymphatic vessels of the dura mater: a new discovery?J Anat. 2015 Nov;227(5):702-3. doi: 10.1111/joa.12381. Epub 2015 Sep 18. J Anat. 2015. PMID: 26383824 Free PMC article. No abstract available.
-
Lymphatics in the Brain?!Neurosurgery. 2016 Feb;78(2):N14. doi: 10.1227/01.neu.0000479890.79747.0d. Neurosurgery. 2016. PMID: 26779792 No abstract available.
Similar articles
-
Lymphatic vessels of the dura mater: a new discovery?J Anat. 2015 Nov;227(5):702-3. doi: 10.1111/joa.12381. Epub 2015 Sep 18. J Anat. 2015. PMID: 26383824 Free PMC article. No abstract available.
-
Implications of the discovery of brain lymphatic pathways.Lancet Neurol. 2015 Oct;14(10):977-9. doi: 10.1016/S1474-4422(15)00221-5. Lancet Neurol. 2015. PMID: 26376966 Free PMC article. No abstract available.
-
Lymphatics in the Brain?!Neurosurgery. 2016 Feb;78(2):N14. doi: 10.1227/01.neu.0000479890.79747.0d. Neurosurgery. 2016. PMID: 26779792 No abstract available.
-
The Lymphatic System In The Brain Clearance Mechanisms - New Therapeutic Perspectives For Alzheimer's Disease.Curr Neuropharmacol. 2023;21(2):380-391. doi: 10.2174/1570159X20666220411091332. Curr Neuropharmacol. 2023. PMID: 35410605 Free PMC article. Review.
-
[Lymphatic system in central nervous system].Med Sci (Paris). 2019 Jan;35(1):55-61. doi: 10.1051/medsci/2018309. Epub 2019 Jan 23. Med Sci (Paris). 2019. PMID: 30672459 Review. French.
Cited by
-
Repetitive transcranial magnetic stimulation increases the brain's drainage efficiency in a mouse model of Alzheimer's disease.Acta Neuropathol Commun. 2021 Jun 2;9(1):102. doi: 10.1186/s40478-021-01198-3. Acta Neuropathol Commun. 2021. PMID: 34078467 Free PMC article.
-
Why do central nervous system barriers host a diverse immune landscape?Trends Immunol. 2024 Oct;45(10):738-749. doi: 10.1016/j.it.2024.08.009. Epub 2024 Sep 18. Trends Immunol. 2024. PMID: 39299888 Review.
-
Comparative DNA Methylation Profiling Reveals an Immunoepigenetic Signature of HIV-related Cognitive Impairment.Sci Rep. 2016 Sep 15;6:33310. doi: 10.1038/srep33310. Sci Rep. 2016. PMID: 27629381 Free PMC article.
-
Clearance of erythrocytes from the subarachnoid space through cribriform plate lymphatics in female mice.EBioMedicine. 2024 Sep;107:105295. doi: 10.1016/j.ebiom.2024.105295. Epub 2024 Aug 22. EBioMedicine. 2024. PMID: 39178745 Free PMC article.
-
Cellular Origins of the Lymphatic Endothelium: Implications for Cancer Lymphangiogenesis.Front Physiol. 2020 Sep 24;11:577584. doi: 10.3389/fphys.2020.577584. eCollection 2020. Front Physiol. 2020. PMID: 33071831 Free PMC article. Review.
References
-
- Choi I., Chung H.K., Ramu S., Lee H.N., Kim K.E., Lee S., Yoo J., Choi D., Lee Y.S., Aguilar B., and Hong Y.-K.. 2011. Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood. 117:362–365. 10.1182/blood-2010-07-298562 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

