Deletion of a kinesin I motor unmasks a mechanism of homeostatic branching control by neurotrophin-3
- PMID: 26076409
- PMCID: PMC4467164
- DOI: 10.7554/eLife.05061
Deletion of a kinesin I motor unmasks a mechanism of homeostatic branching control by neurotrophin-3
Abstract
Development and function of highly polarized cells such as neurons depend on microtubule-associated intracellular transport, but little is known about contributions of specific molecular motors to the establishment of synaptic connections. In this study, we investigated the function of the Kinesin I heavy chain Kif5aa during retinotectal circuit formation in zebrafish. Targeted disruption of Kif5aa does not affect retinal ganglion cell differentiation, and retinal axons reach their topographically correct targets in the tectum, albeit with a delay. In vivo dynamic imaging showed that anterograde transport of mitochondria is impaired, as is synaptic transmission. Strikingly, disruption of presynaptic activity elicits upregulation of Neurotrophin-3 (Ntf3) in postsynaptic tectal cells. This in turn promotes exuberant branching of retinal axons by signaling through the TrkC receptor (Ntrk3). Thus, our study has uncovered an activity-dependent, retrograde signaling pathway that homeostatically controls axonal branching.
Keywords: axonal development; neuroscience; neurotrophic signaling; visual system; zebrafish.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


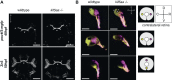


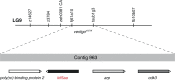
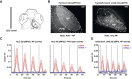
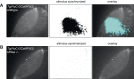







Similar articles
-
Unique function of Kinesin Kif5A in localization of mitochondria in axons.J Neurosci. 2014 Oct 29;34(44):14717-32. doi: 10.1523/JNEUROSCI.2770-14.2014. J Neurosci. 2014. PMID: 25355224 Free PMC article.
-
Kinesin-12 influences axonal growth during zebrafish neural development.Cytoskeleton (Hoboken). 2014 Oct;71(10):555-63. doi: 10.1002/cm.21193. Epub 2014 Oct 30. Cytoskeleton (Hoboken). 2014. PMID: 25250533 Free PMC article.
-
PlexinA4 is necessary as a downstream target of Islet2 to mediate Slit signaling for promotion of sensory axon branching.Development. 2004 Aug;131(15):3705-15. doi: 10.1242/dev.01228. Epub 2004 Jun 30. Development. 2004. PMID: 15229183
-
Fluorescent proteins in zebrafish cell and developmental biology.Methods Cell Biol. 2008;85:219-41. doi: 10.1016/S0091-679X(08)85010-8. Methods Cell Biol. 2008. PMID: 18155465 Review.
-
Time-lapse imaging of neural development: zebrafish lead the way into the fourth dimension.Genesis. 2011 Jul;49(7):534-45. doi: 10.1002/dvg.20729. Epub 2011 Apr 2. Genesis. 2011. PMID: 21305690 Free PMC article. Review.
Cited by
-
Methodological advances in imaging intravital axonal transport.F1000Res. 2017 Mar 1;6:200. doi: 10.12688/f1000research.10433.1. eCollection 2017. F1000Res. 2017. PMID: 28344778 Free PMC article. Review.
-
Regulation of long-distance transport of mitochondria along microtubules.Cell Mol Life Sci. 2018 Jan;75(2):163-176. doi: 10.1007/s00018-017-2590-1. Epub 2017 Jul 12. Cell Mol Life Sci. 2018. PMID: 28702760 Free PMC article. Review.
-
Dominantly acting KIF5B variants with pleiotropic cellular consequences cause variable clinical phenotypes.Hum Mol Genet. 2023 Jan 13;32(3):473-488. doi: 10.1093/hmg/ddac213. Hum Mol Genet. 2023. PMID: 36018820 Free PMC article.
-
KIF5A regulates axonal repair and time-dependent axonal transport of SFPQ granules and mitochondria in human motor neurons.bioRxiv [Preprint]. 2024 Sep 11:2024.09.06.611684. doi: 10.1101/2024.09.06.611684. bioRxiv. 2024. Update in: Neurobiol Dis. 2025 Jan;204:106759. doi: 10.1016/j.nbd.2024.106759. PMID: 39314491 Free PMC article. Updated. Preprint.
-
Mechanosensory neurons control the timing of spinal microcircuit selection during locomotion.Elife. 2017 Jun 19;6:e25260. doi: 10.7554/eLife.25260. Elife. 2017. PMID: 28623664 Free PMC article.
References
-
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Mackli JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SS, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. Optimization of a GCaMP calcium indicator for neural activity imaging. The Journal of Neuroscience. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

