Synthesis, DNA Binding, and Antiproliferative Activity of Novel Acridine-Thiosemicarbazone Derivatives
- PMID: 26068233
- PMCID: PMC4490484
- DOI: 10.3390/ijms160613023
Synthesis, DNA Binding, and Antiproliferative Activity of Novel Acridine-Thiosemicarbazone Derivatives
Abstract
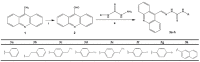
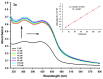
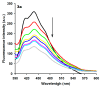
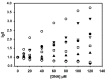
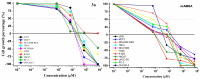
In this work, the acridine nucleus was used as a lead-compound for structural modification by adding different substituted thiosemicarbazide moieties. Eight new (Z)-2-(acridin-9-ylmethylene)-N-phenylhydrazinecarbothioamide derivatives (3a-h) were synthesized, their antiproliferative activities were evaluated, and DNA binding properties were performed with calf thymus DNA (ctDNA) by electronic absorption and fluorescence spectroscopies. Both hyperchromic and hypochromic effects, as well as red or blue shifts were demonstrated by addition of ctDNA to the derivatives. The calculated binding constants ranged from 1.74 × 10(4) to 1.0 × 10(6) M(-1) and quenching constants from -0.2 × 10(4) to 2.18 × 10(4) M(-1) indicating high affinity to ctDNA base pairs. The most efficient compound in binding to ctDNA in vitro was (Z)-2-(acridin-9-ylmethylene)-N- (4-chlorophenyl) hydrazinecarbothioamide (3f), while the most active compound in antiproliferative assay was (Z)-2-(acridin-9-ylmethylene)-N-phenylhydrazinecarbothioamide (3a). There was no correlation between DNA-binding and in vitro antiproliferative activity, but the results suggest that DNA binding can be involved in the biological activity mechanism. This study may guide the choice of the size and shape of the intercalating part of the ligand and the strategic selection of substituents that increase DNA-binding or antiproliferative properties.
Keywords: DNA binding; acridine; antiproliferative; thiosemicarbazone.
Figures
Similar articles
-
Topoisomerase inhibition and albumin interaction studies of acridine-thiosemicarbazone derivatives.Int J Biol Macromol. 2019 Oct 1;138:582-589. doi: 10.1016/j.ijbiomac.2019.07.097. Epub 2019 Jul 16. Int J Biol Macromol. 2019. PMID: 31323270
-
Novel 4-quinoline-thiosemicarbazone derivatives: Synthesis, antiproliferative activity, in vitro and in silico biomacromolecule interaction studies and topoisomerase inhibition.Eur J Med Chem. 2019 Nov 15;182:111592. doi: 10.1016/j.ejmech.2019.111592. Epub 2019 Aug 6. Eur J Med Chem. 2019. PMID: 31421632
-
Synthesis, DNA-binding and antiproliferative properties of acridine and 5-methylacridine derivatives.Molecules. 2012 Jun 8;17(6):7067-82. doi: 10.3390/molecules17067067. Molecules. 2012. PMID: 22683895 Free PMC article.
-
A Review on Acridines as Antiproliferative Agents.Mini Rev Med Chem. 2022;22(21):2769-2798. doi: 10.2174/1389557522666220511125744. Mini Rev Med Chem. 2022. PMID: 35546777 Review.
-
Synthesis, structure-activity relationship and biological activity of acridine derivatives as potent MDR-reversing agents.Curr Med Chem. 2013;20(32):4070-9. doi: 10.2174/09298673113209990187. Curr Med Chem. 2013. PMID: 23895691 Review.
Cited by
-
Bivalent Ni(II), Co(II) and Cu(II) complexes of [(E)-[(2-methyl-1,3-thiazol-5-yl)methylidene]amino]thiourea: synthesis, spectral characterization, DNA and in-vitro anti-bacterial studies.Heliyon. 2021 Apr 22;7(4):e06838. doi: 10.1016/j.heliyon.2021.e06838. eCollection 2021 Apr. Heliyon. 2021. PMID: 33997386 Free PMC article.
-
Enhanced Efficacy of Thiosemicarbazone Derivative-Encapsulated Fibrin Liposomes against Candidiasis in Murine Model.Pharmaceutics. 2021 Mar 4;13(3):333. doi: 10.3390/pharmaceutics13030333. Pharmaceutics. 2021. PMID: 33806702 Free PMC article.
-
Novel tetrahydroacridine derivatives with iodobenzoic moieties induce G0/G1 cell cycle arrest and apoptosis in A549 non-small lung cancer and HT-29 colorectal cancer cells.Mol Cell Biochem. 2019 Oct;460(1-2):123-150. doi: 10.1007/s11010-019-03576-x. Epub 2019 Jul 16. Mol Cell Biochem. 2019. PMID: 31313023 Free PMC article.
-
Synthesis and evaluation of anticancer activity of new 9-acridinyl amino acid derivatives.RSC Med Chem. 2020 Feb 14;11(3):378-386. doi: 10.1039/c9md00597h. eCollection 2020 Mar 1. RSC Med Chem. 2020. PMID: 33479643 Free PMC article.
-
5-Methoxyisatin N(4)-Pyrrolidinyl Thiosemicarbazone (MeOIstPyrd) Restores Mutant p53 and Inhibits the Growth of Skin Cancer Cells, In Vitro.ACS Omega. 2023 Aug 24;8(35):31998-32016. doi: 10.1021/acsomega.3c03824. eCollection 2023 Sep 5. ACS Omega. 2023. PMID: 37692215 Free PMC article.
References
-
- Maaloum M., Muller P., Harlepp S. DNA-intercalator interactions: Structural and physical analysis using atomic force microscopy in solution. Soft Matter. 2013;9:11233–11240. doi: 10.1039/c3sm52082j. - DOI
-
- Kumar R., Kaur M., Kumari M. Acridine: A versatile heterocyclic nucleus. Acta Pol. Pharm. 2012;69:3–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

