Ganglioside accumulation in activated glia in the developing brain: comparison between WT and GalNAcT KO mice
- PMID: 26063460
- PMCID: PMC4513985
- DOI: 10.1194/jlr.M056580
Ganglioside accumulation in activated glia in the developing brain: comparison between WT and GalNAcT KO mice
Abstract
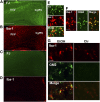
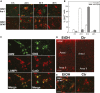
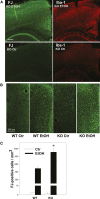
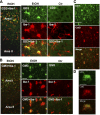
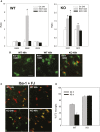
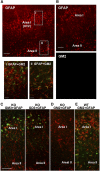
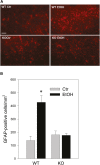
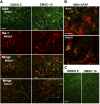
Our previous studies have shown accumulation of GM2 ganglioside during ethanol-induced neurodegeneration in the developing brain, and GM2 elevation has also been reported in other brain injuries and neurodegenerative diseases. Using GM2/GD2 synthase KO mice lacking GM2/GD2 and downstream gangliosides, the current study explored the significance of GM2 elevation in WT mice. Immunohistochemical studies indicated that ethanol-induced acute neurodegeneration in postnatal day 7 (P7) WT mice was associated with GM2 accumulation in the late endosomes/lysosomes of both phagocytic microglia and increased glial fibrillary acidic protein (GFAP)-positive astrocytes. However, in KO mice, although ethanol induced robust neurodegeneration and accumulation of GD3 and GM3 in the late endosomes/lysosomes of phagocytic microglia, it did not increase the number of GFAP-positive astrocytes, and the accumulation of GD3/GM3 in astrocytes was minimal. Not only ethanol, but also DMSO, induced GM2 elevation in activated microglia and astrocytes along with neurodegeneration in P7 WT mice, while lipopolysaccharide, which did not induce significant neurodegeneration, caused GM2 accumulation mainly in lysosomes of activated astrocytes. Thus, GM2 elevation is associated with activation of microglia and astrocytes in the injured developing brain, and GM2, GD2, or other downstream gangliosides may regulate astroglial responses in ethanol-induced neurodegeneration.
Keywords: GM2/GD2 synthase knockout mice; activated microglia; astrocyte; brain lipids; ceramide; ethanol; inflammation; lipopolysaccharide; triglycerides; wild-type mice.
Copyright © 2015 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Elevation of GM2 ganglioside during ethanol-induced apoptotic neurodegeneration in the developing mouse brain.J Neurochem. 2012 May;121(4):649-61. doi: 10.1111/j.1471-4159.2012.07710.x. Epub 2012 Mar 20. J Neurochem. 2012. PMID: 22372857 Free PMC article.
-
Gangliosides are essential in the protection of inflammation and neurodegeneration via maintenance of lipid rafts: elucidation by a series of ganglioside-deficient mutant mice.J Neurochem. 2011 Mar;116(5):926-35. doi: 10.1111/j.1471-4159.2010.07067.x. Epub 2011 Jan 12. J Neurochem. 2011. PMID: 21214571
-
Ganglioside GD3 in radial glia and astrocytes in situ in brains of young and adult mice.J Neurosci Res. 1996 Oct 1;46(1):18-23. doi: 10.1002/(SICI)1097-4547(19961001)46:1<18::AID-JNR3>3.0.CO;2-I. J Neurosci Res. 1996. PMID: 8892101
-
Beta1,4-N-acetylgalactosaminyltransferase--GM2/GD2 synthase: a key enzyme to control the synthesis of brain-enriched complex gangliosides.Biochim Biophys Acta. 2002 Dec 19;1573(3):356-62. doi: 10.1016/s0304-4165(02)00403-8. Biochim Biophys Acta. 2002. PMID: 12417418 Review.
-
Ethanol-Induced Neurodegeneration and Glial Activation in the Developing Brain.Brain Sci. 2016 Aug 16;6(3):31. doi: 10.3390/brainsci6030031. Brain Sci. 2016. PMID: 27537918 Free PMC article. Review.
Cited by
-
Lipid rafts and neurodegeneration: structural and functional roles in physiologic aging and neurodegenerative diseases.J Lipid Res. 2020 May;61(5):636-654. doi: 10.1194/jlr.TR119000427. Epub 2019 Dec 23. J Lipid Res. 2020. PMID: 31871065 Free PMC article. Review.
-
Apolipoprotein E4 genotype compromises brain exosome production.Brain. 2019 Jan 1;142(1):163-175. doi: 10.1093/brain/awy289. Brain. 2019. PMID: 30496349 Free PMC article.
-
Topical Coenzyme Q10 demonstrates mitochondrial-mediated neuroprotection in a rodent model of ocular hypertension.Mitochondrion. 2017 Sep;36:114-123. doi: 10.1016/j.mito.2017.05.010. Epub 2017 May 24. Mitochondrion. 2017. PMID: 28549843 Free PMC article.
-
Single Cerebral Organoid Mass Spectrometry of Cell-Specific Protein and Glycosphingolipid Traits.Anal Chem. 2023 Feb 14;95(6):3160-3167. doi: 10.1021/acs.analchem.2c00981. Epub 2023 Feb 1. Anal Chem. 2023. PMID: 36724094 Free PMC article.
-
Involvement of AMP-activated protein kinase in neuroinflammation and neurodegeneration in the adult and developing brain.Int J Dev Neurosci. 2019 Oct;77:48-59. doi: 10.1016/j.ijdevneu.2019.01.007. Epub 2019 Jan 29. Int J Dev Neurosci. 2019. PMID: 30707928 Free PMC article. Review.
References
-
- Ikonomidou C., Bittigau P., Ishimaru M. J., Wozniak D. F., Koch C., Genz K., Price M. T., Stefovska V., Horster F., Tenkova T., et al. 2000. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 287: 1056–1060. - PubMed
-
- Goodlett C. R., Leo J. T., O’Callaghan J. P., Mahoney J. C., West J. R. 1993. Transient cortical astrogliosis induced by alcohol exposure during the neonatal brain growth spurt in rats. Brain Res. Dev. Brain Res. 72: 85–97. - PubMed
-
- Kane C. J., Phelan K. D., Han L., Smith R. R., Xie J., Douglas J. C., Drew P. D. 2011. Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisome proliferator-activated receptor-gamma agonists. Brain Behav. Immun. 25(Suppl 1): S137–S145. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

