The neuroleptic drug pimozide inhibits stem-like cell maintenance and tumorigenicity in hepatocellular carcinoma
- PMID: 26061710
- PMCID: PMC5392272
- DOI: 10.18632/oncotarget.4307
The neuroleptic drug pimozide inhibits stem-like cell maintenance and tumorigenicity in hepatocellular carcinoma
Abstract
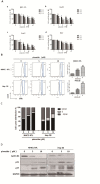
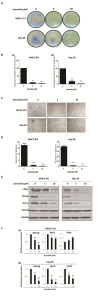
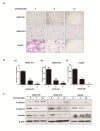
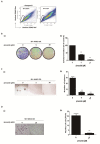
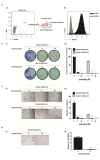
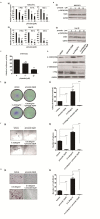
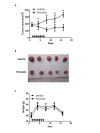
Drug repurposing is currently an important approach for accelerating drug discovery and development for clinical use. Hepatocellular carcinoma (HCC) presents drug resistance to chemotherapy, and the prognosis is poor due to the existence of liver cancer stem-like cells. In this study, we investigated the effect of the neuroleptic agent pimozide to inhibit stem-like cell maintenance and tumorigenicity in HCC. Our results showed that pimozide functioned as an anti-cancer drug in HCC cells or stem-like cells. Pimozide inhibited cell proliferation and sphere formation capacities in HCC cells by inducing G0/G1 phase cell cycle arrest, as well as inhibited HCC cell migration. Surprisingly, pimozide inhibited the maintenance and tumorigenicity of HCC stem-like cells, particularly the side population (SP) or CD133-positive cells, as evaluated by colony formation, sphere formation and transwell migration assays. Furthermore, pimozide was found to suppress STAT3 activity in HCC cells by attenuating STAT3-dependent luciferase activity and down-regulating the transcription levels of downstream genes of STAT3 signaling. Moreover, pimozide reversed the stem-like cell tumorigenic phenotypes induced by IL-6 treatment in HCC cells. Further, the antitumor effect of pimozide was also proved in the nude mice HCC xenograft model. In short, the anti-psychotic agent pimozide may act as a novel potential anti-tumor agent in treating advanced HCC.
Keywords: STAT3 inhibitor; STAT3 signaling; hepatic cancer stem-like cells; pimozide; self-renewal.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
The antipsychotic drug pimozide inhibits cell growth in prostate cancer through suppression of STAT3 activation.Int J Oncol. 2016 Jan;48(1):322-8. doi: 10.3892/ijo.2015.3229. Epub 2015 Nov 4. Int J Oncol. 2016. PMID: 26549437
-
WM130 preferentially inhibits hepatic cancer stem-like cells by suppressing AKT/GSK3β/β-catenin signaling pathway.Oncotarget. 2016 Nov 29;7(48):79544-79556. doi: 10.18632/oncotarget.12822. Oncotarget. 2016. PMID: 27783993 Free PMC article.
-
Antipsychotic agent pimozide promotes reversible proliferative suppression by inducing cellular quiescence in liver cancer.Oncol Rep. 2019 Sep;42(3):1101-1109. doi: 10.3892/or.2019.7229. Epub 2019 Jul 11. Oncol Rep. 2019. PMID: 31322218 Free PMC article.
-
Inhibition of cancer stem cells promoted by Pimozide.Clin Exp Pharmacol Physiol. 2019 Feb;46(2):116-125. doi: 10.1111/1440-1681.13049. Epub 2018 Nov 28. Clin Exp Pharmacol Physiol. 2019. PMID: 30383889 Review.
-
Potential targets and therapeutics for cancer stem cell-based therapy against drug resistance in hepatocellular carcinoma.Drug Resist Updat. 2024 May;74:101084. doi: 10.1016/j.drup.2024.101084. Epub 2024 Apr 16. Drug Resist Updat. 2024. PMID: 38640592 Review.
Cited by
-
Involvement of STAT5 in Oncogenesis.Biomedicines. 2020 Aug 28;8(9):316. doi: 10.3390/biomedicines8090316. Biomedicines. 2020. PMID: 32872372 Free PMC article. Review.
-
Pimozide Inhibits the Human Prostate Cancer Cells Through the Generation of Reactive Oxygen Species.Front Pharmacol. 2020 Jan 16;10:1517. doi: 10.3389/fphar.2019.01517. eCollection 2019. Front Pharmacol. 2020. PMID: 32009948 Free PMC article.
-
Repurposing the anti-malarial drug artesunate as a novel therapeutic agent for metastatic renal cell carcinoma due to its attenuation of tumor growth, metastasis, and angiogenesis.Oncotarget. 2015 Oct 20;6(32):33046-64. doi: 10.18632/oncotarget.5422. Oncotarget. 2015. PMID: 26426994 Free PMC article.
-
Suppressing STAT5 signaling affects osteosarcoma growth and stemness.Cell Death Dis. 2020 Feb 24;11(2):149. doi: 10.1038/s41419-020-2335-1. Cell Death Dis. 2020. PMID: 32094348 Free PMC article.
-
Drug rechanneling: A novel paradigm for cancer treatment.Semin Cancer Biol. 2021 Jan;68:279-290. doi: 10.1016/j.semcancer.2020.03.011. Epub 2020 May 11. Semin Cancer Biol. 2021. PMID: 32437876 Free PMC article. Review.
References
-
- Boguski MS, Mandl KD, Sukhatme VP. Drug discovery. Repurposing with a difference. Science. 2009;324(5933):1394–1395. - PubMed
-
- Carley DW. Drug repurposing: identify, develop and commercialize new uses for existing or abandoned drugs. Part I. IDrugs. 2005;8:306–309. - PubMed
-
- Carley DW. Drug repurposing: identify, develop and commercialize new uses for existing or abandoned drugs. Part II. IDrugs. 2005;8:310–313. - PubMed
-
- Blatt J, Corey SJ. Drug repurposing in pediatrics and pediatric hematology oncology. Drug Discov Today. 2013;18:4–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

