The Intranasal Application of Zanamivir and Carrageenan Is Synergistically Active against Influenza A Virus in the Murine Model
- PMID: 26053018
- PMCID: PMC4459876
- DOI: 10.1371/journal.pone.0128794
The Intranasal Application of Zanamivir and Carrageenan Is Synergistically Active against Influenza A Virus in the Murine Model
Abstract
Background: Carrageenan is a clinically proven and marketed compound for the treatment of viral upper respiratory tract infections. As infections caused by influenza virus are often accompanied by infections with other respiratory viruses the combination of a specific anti-influenza compound with the broadly active antiviral polymer has huge potential for the treatment of respiratory infections. Thus, the combination of the specific anti-influenza drug Zanamivir together with carrageenan in a formulation suitable for intranasal application was evaluated in-vitro and in-vivo.
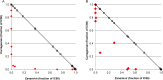
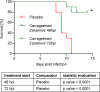
Principal findings: We show in-vitro that carrageenan and Zanamivir act synergistically against several influenza A virus strains (H1N1(09)pdm, H3N2, H5N1, H7N7). Moreover, we demonstrate in a lethal influenza model with a low pathogenic H7N7 virus (HA closely related to the avian influenza A(H7N9) virus) and a H1N1(09)pdm influenza virus in C57BL/6 mice that the combined use of both compounds significantly increases survival of infected animals in comparison with both mono-therapies or placebo. Remarkably, this benefit is maintained even when the treatment starts up to 72 hours post infection.
Conclusion: A nasal spray containing carrageenan and Zanamivir should therefore be tested for prevention and treatment of uncomplicated influenza in clinical trials.
Conflict of interest statement
Figures

Similar articles
-
Efficacy of zanamivir against avian influenza A viruses that possess genes encoding H5N1 internal proteins and are pathogenic in mammals.Antimicrob Agents Chemother. 2001 Apr;45(4):1216-24. doi: 10.1128/AAC.45.4.1216-1224.2001. Antimicrob Agents Chemother. 2001. PMID: 11257037 Free PMC article.
-
A zanamivir dimer with prophylactic and enhanced therapeutic activity against influenza viruses.J Antimicrob Chemother. 2014 Aug;69(8):2164-74. doi: 10.1093/jac/dku127. Epub 2014 Apr 28. J Antimicrob Chemother. 2014. PMID: 24777908 Free PMC article.
-
Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses.Antimicrob Agents Chemother. 2001 Oct;45(10):2723-32. doi: 10.1128/AAC.45.10.2723-2732.2001. Antimicrob Agents Chemother. 2001. PMID: 11557461 Free PMC article.
-
Neuraminidase inhibitor resistance in influenza viruses.J Med Virol. 2007 Oct;79(10):1577-86. doi: 10.1002/jmv.20951. J Med Virol. 2007. PMID: 17705169 Review.
-
Laninamivir octanoate: a new long-acting neuraminidase inhibitor for the treatment of influenza.Expert Rev Anti Infect Ther. 2011 Oct;9(10):851-7. doi: 10.1586/eri.11.112. Expert Rev Anti Infect Ther. 2011. PMID: 21973296 Review.
Cited by
-
Viral inhibitors derived from macroalgae, microalgae, and cyanobacteria: A review of antiviral potential throughout pathogenesis.Algal Res. 2021 Jul;57:102331. doi: 10.1016/j.algal.2021.102331. Epub 2021 May 18. Algal Res. 2021. PMID: 34026476 Free PMC article.
-
Recent Advances in Chemically-Modified and Hybrid Carrageenan-Based Platforms for Drug Delivery, Wound Healing, and Tissue Engineering.Polymers (Basel). 2021 May 26;13(11):1744. doi: 10.3390/polym13111744. Polymers (Basel). 2021. PMID: 34073518 Free PMC article. Review.
-
Advances in Research on Antiviral Activities of Sulfated Polysaccharides from Seaweeds.Pharmaceuticals (Basel). 2022 May 6;15(5):581. doi: 10.3390/ph15050581. Pharmaceuticals (Basel). 2022. PMID: 35631407 Free PMC article. Review.
-
Antiviral Strategies Using Natural Source-Derived Sulfated Polysaccharides in the Light of the COVID-19 Pandemic and Major Human Pathogenic Viruses.Viruses. 2021 Dec 24;14(1):35. doi: 10.3390/v14010035. Viruses. 2021. PMID: 35062238 Free PMC article. Review.
-
Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta.Int J Mol Sci. 2021 Dec 8;22(24):13202. doi: 10.3390/ijms222413202. Int J Mol Sci. 2021. PMID: 34947999 Free PMC article.
References
-
- WHO. Influenza (seasonal). 2009;1–3.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

