Ion channel macromolecular complexes in cardiomyocytes: roles in sudden cardiac death
- PMID: 26044251
- PMCID: PMC4471480
- DOI: 10.1161/CIRCRESAHA.116.305017
Ion channel macromolecular complexes in cardiomyocytes: roles in sudden cardiac death
Abstract
The movement of ions across specific channels embedded on the membrane of individual cardiomyocytes is crucial for the generation and propagation of the cardiac electric impulse. Emerging evidence over the past 20 years strongly suggests that the normal electric function of the heart is the result of dynamic interactions of membrane ion channels working in an orchestrated fashion as part of complex molecular networks. Such networks work together with exquisite temporal precision to generate each action potential and contraction. Macromolecular complexes play crucial roles in transcription, translation, oligomerization, trafficking, membrane retention, glycosylation, post-translational modification, turnover, function, and degradation of all cardiac ion channels known to date. In addition, the accurate timing of each cardiac beat and contraction demands, a comparable precision on the assembly and organizations of sodium, calcium, and potassium channel complexes within specific subcellular microdomains, where physical proximity allows for prompt and efficient interaction. This review article, part of the Compendium on Sudden Cardiac Death, discusses the major issues related to the role of ion channel macromolecular assemblies in normal cardiac electric function and the mechanisms of arrhythmias leading to sudden cardiac death. It provides an idea of how these issues are being addressed in the laboratory and in the clinic, which important questions remain unanswered, and what future research will be needed to improve knowledge and advance therapy.
Keywords: arrhythmias, cardiac; death, sudden, cardiac; ion channels; multiprotein complexes.
© 2015 American Heart Association, Inc.
Figures
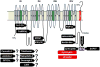

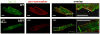




Similar articles
-
Cardiac voltage-gated calcium channel macromolecular complexes.Biochim Biophys Acta. 2016 Jul;1863(7 Pt B):1806-12. doi: 10.1016/j.bbamcr.2015.12.014. Epub 2015 Dec 18. Biochim Biophys Acta. 2016. PMID: 26707467 Review.
-
Protein assemblies of sodium and inward rectifier potassium channels control cardiac excitability and arrhythmogenesis.Am J Physiol Heart Circ Physiol. 2015 Jun 15;308(12):H1463-73. doi: 10.1152/ajpheart.00176.2015. Epub 2015 Apr 10. Am J Physiol Heart Circ Physiol. 2015. PMID: 25862830 Free PMC article. Review.
-
[Anchoring proteins and cardiac sudden death: how and why?].Med Sci (Paris). 2004 Apr;20(4):437-41. doi: 10.1051/medsci/2004204437. Med Sci (Paris). 2004. PMID: 15124116 Review. French.
-
Kir2.1-NaV1.5 channelosome and its role in arrhythmias in inheritable cardiac diseases.Heart Rhythm. 2024 May;21(5):630-646. doi: 10.1016/j.hrthm.2024.01.017. Epub 2024 Jan 18. Heart Rhythm. 2024. PMID: 38244712 Review.
-
[Research Progress of the Correlation between Caveolin and Unexpected Sudden Cardiac Death].Fa Yi Xue Za Zhi. 2017 Jun;33(3):284-288. doi: 10.3969/j.issn.1004-5619.2017.03.015. Epub 2017 Jun 25. Fa Yi Xue Za Zhi. 2017. PMID: 29230996 Review. Chinese.
Cited by
-
Models of the cardiac L-type calcium current: A quantitative review.WIREs Mech Dis. 2023 Jan;15(1):e1581. doi: 10.1002/wsbm.1581. Epub 2022 Aug 26. WIREs Mech Dis. 2023. PMID: 36028219 Free PMC article. Review.
-
Cell-Adhesion Properties of β-Subunits in the Regulation of Cardiomyocyte Sodium Channels.Biomolecules. 2020 Jul 1;10(7):989. doi: 10.3390/biom10070989. Biomolecules. 2020. PMID: 32630316 Free PMC article. Review.
-
Transcriptomic analyses of murine ventricular cardiomyocytes.Sci Data. 2018 Aug 21;5:180170. doi: 10.1038/sdata.2018.170. Sci Data. 2018. PMID: 30129933 Free PMC article.
-
Small-conductance calcium-activated potassium channels in the heart: expression, regulation and pathological implications.Philos Trans R Soc Lond B Biol Sci. 2023 Jun 19;378(1879):20220171. doi: 10.1098/rstb.2022.0171. Epub 2023 May 1. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37122223 Free PMC article. Review.
-
Detecting early myocardial ischemia in rat heart by MALDI imaging mass spectrometry.Sci Rep. 2021 Mar 4;11(1):5135. doi: 10.1038/s41598-021-84523-z. Sci Rep. 2021. PMID: 33664384 Free PMC article.
References
-
- Patwardhan A, Ashton A, Brandt R, Butcher S, Carzaniga R, Chiu W, Collinson L, Doux P, Duke E, Ellisman MH, Franken E, Grunewald K, Heriche JK, Koster A, Kuhlbrandt W, Lagerstedt I, Larabell C, Lawson CL, Saibil HR, Sanz-Garcia E, Subramaniam S, Verkade P, Swedlow JR, Kleywegt GJ. A 3d cellular context for the macromolecular world. Nature structural & molecular biology. 2014;21:841–845. - PMC - PubMed
-
- Cebecauer M, Spitaler M, Serge A, Magee AI. Signalling complexes and clusters: Functional advantages and methodological hurdles. J Cell Sci. 2010;123:309–320. - PubMed
-
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

