Drug-induced regeneration in adult mice
- PMID: 26041709
- PMCID: PMC4687906
- DOI: 10.1126/scitranslmed.3010228
Drug-induced regeneration in adult mice
Abstract
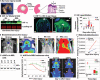
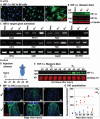
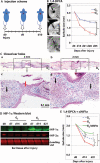
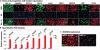
Whereas amphibians regenerate lost appendages spontaneously, mammals generally form scars over the injury site through the process of wound repair. The MRL mouse strain is an exception among mammals because it shows a spontaneous regenerative healing trait and so can be used to investigate proregenerative interventions in mammals. We report that hypoxia-inducible factor 1α (HIF-1α) is a central molecule in the process of regeneration in adult MRL mice. The degradation of HIF-1α protein, which occurs under normoxic conditions, is mediated by prolyl hydroxylases (PHDs). We used the drug 1,4-dihydrophenonthrolin-4-one-3-carboxylic acid (1,4-DPCA), a PHD inhibitor, to stabilize constitutive expression of HIF-1α protein. A locally injectable hydrogel containing 1,4-DPCA was designed to achieve controlled delivery of the drug over 4 to 10 days. Subcutaneous injection of the 1,4-DPCA/hydrogel into Swiss Webster mice that do not show a regenerative phenotype increased stable expression of HIF-1α protein over 5 days, providing a functional measure of drug release in vivo. Multiple peripheral subcutaneous injections of the 1,4-DPCA/hydrogel over a 10-day period led to regenerative wound healing in Swiss Webster mice after ear hole punch injury. Increased expression of the HIF-1α protein may provide a starting point for future studies on regeneration in mammals.
Copyright © 2015, American Association for the Advancement of Science.
Figures

Similar articles
-
An injectable hydrogel-formulated inhibitor of prolyl-4-hydroxylase promotes T regulatory cell recruitment and enhances alveolar bone regeneration during resolution of experimental periodontitis.FASEB J. 2020 Oct;34(10):13726-13740. doi: 10.1096/fj.202001248R. Epub 2020 Aug 19. FASEB J. 2020. PMID: 32812255 Free PMC article.
-
Thermosensitive Hydrogel with Programmable, Self-Regulated HIF-1α Stabilizer Release for Myocardial Infarction Treatment.Adv Sci (Weinh). 2024 Nov;11(43):e2408013. doi: 10.1002/advs.202408013. Epub 2024 Sep 23. Adv Sci (Weinh). 2024. PMID: 39308185 Free PMC article.
-
Synergistic effect of ROS-generating polydopamine on drug-induced bone tissue regeneration.Nanoscale. 2024 Nov 7;16(43):20118-20130. doi: 10.1039/d4nr02887b. Nanoscale. 2024. PMID: 39405040
-
Oxygen, Metabolism, and Regeneration: Lessons from Mice.Trends Mol Med. 2017 Nov;23(11):1024-1036. doi: 10.1016/j.molmed.2017.08.008. Epub 2017 Oct 5. Trends Mol Med. 2017. PMID: 28988849 Free PMC article. Review.
-
Drug delivery and epimorphic salamander-type mouse regeneration: A full parts and labor plan.Adv Drug Deliv Rev. 2018 Apr;129:254-261. doi: 10.1016/j.addr.2018.02.006. Epub 2018 Mar 7. Adv Drug Deliv Rev. 2018. PMID: 29524586 Free PMC article. Review.
Cited by
-
Real-time physiological measurements of oxygen using a non-invasive self-referencing optical fiber microsensor.Nat Protoc. 2020 Feb;15(2):207-235. doi: 10.1038/s41596-019-0231-x. Epub 2020 Jan 10. Nat Protoc. 2020. PMID: 31925402 Free PMC article.
-
Limitations and Promise of Retinal Tissue From Human Pluripotent Stem Cells for Developing Therapies of Blindness.Front Cell Neurosci. 2020 Sep 10;14:179. doi: 10.3389/fncel.2020.00179. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33132839 Free PMC article. Review.
-
An MMP-degradable and conductive hydrogel to stabilize HIF-1α for recovering cardiac functions.Theranostics. 2022 Jan 1;12(1):127-142. doi: 10.7150/thno.63481. eCollection 2022. Theranostics. 2022. PMID: 34987638 Free PMC article.
-
Systems genetics identifies a macrophage cholesterol network associated with physiological wound healing.JCI Insight. 2019 Jan 24;4(2):e125736. doi: 10.1172/jci.insight.125736. JCI Insight. 2019. PMID: 30674726 Free PMC article.
-
dbCRSR: a manually curated database for regulation of cancer radiosensitivity.Database (Oxford). 2018 Jan 1;2018:bay049. doi: 10.1093/database/bay049. Database (Oxford). 2018. PMID: 29860480 Free PMC article.
References
-
- Kawasumi A, Sagawa N, Hayashi S, Yokoyama H, Tamura K. Wound healing in mammals and amphibians: Toward limb regeneration in mammals. Curr. Top. Microbiol. Immunol. 2013;367:33–49. - PubMed
-
- Joseph J, Dyson M. Tissue replacement in the rabbit's ear. Br. J. Surg. 1966;53:372–380. - PubMed
-
- Goss RJ, Grimes LN. Tissue interactions in regeneration of rabbit ear holes. Am. Zool. 1972;12:151–157.
-
- Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin. Immunol. Immunopathol. 1998;88:35–45. - PubMed
-
- Kench JA, Russell DM, Fadok VA, Young SK, Worthen GS, Jones-Carson J, Henson JE, Nemazee D. Aberrant wound healing and TGF-β production in the autoimmune-prone MRL/+ mouse. Clin. Immunol. 1999;92:300–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

