ESCRT-III controls nuclear envelope reformation
- PMID: 26040713
- PMCID: PMC4471131
- DOI: 10.1038/nature14503
ESCRT-III controls nuclear envelope reformation
Abstract
During telophase, the nuclear envelope (NE) reforms around daughter nuclei to ensure proper segregation of nuclear and cytoplasmic contents. NE reformation requires the coating of chromatin by membrane derived from the endoplasmic reticulum, and a subsequent annular fusion step to ensure that the formed envelope is sealed. How annular fusion is accomplished is unknown, but it is thought to involve the p97 AAA-ATPase complex and bears a topological equivalence to the membrane fusion event that occurs during the abscission phase of cytokinesis. Here we show that the endosomal sorting complex required for transport-III (ESCRT-III) machinery localizes to sites of annular fusion in the forming NE in human cells, and is necessary for proper post-mitotic nucleo-cytoplasmic compartmentalization. The ESCRT-III component charged multivesicular body protein 2A (CHMP2A) is directed to the forming NE through binding to CHMP4B, and provides an activity essential for NE reformation. Localization also requires the p97 complex member ubiquitin fusion and degradation 1 (UFD1). Our results describe a novel role for the ESCRT machinery in cell division and demonstrate a conservation of the machineries involved in topologically equivalent mitotic membrane remodelling events.
Figures



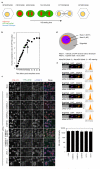
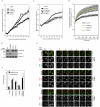
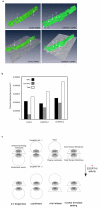
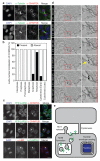
Comment in
-
Cell biology: nuclear dilemma resolved.Nature. 2015 Jun 11;522(7555):159-60. doi: 10.1038/nature14527. Epub 2015 Jun 3. Nature. 2015. PMID: 26040718 No abstract available.
-
CELL BIOLOGY. An ESCRT to seal the envelope.Science. 2015 Jun 19;348(6241):1314-5. doi: 10.1126/science.aac7083. Science. 2015. PMID: 26089496 No abstract available.
Similar articles
-
CC2D1B Coordinates ESCRT-III Activity during the Mitotic Reformation of the Nuclear Envelope.Dev Cell. 2018 Dec 3;47(5):547-563.e6. doi: 10.1016/j.devcel.2018.11.012. Dev Cell. 2018. PMID: 30513301 Free PMC article.
-
Membrane Binding by CHMP7 Coordinates ESCRT-III-Dependent Nuclear Envelope Reformation.Curr Biol. 2016 Oct 10;26(19):2635-2641. doi: 10.1016/j.cub.2016.07.039. Epub 2016 Sep 8. Curr Biol. 2016. PMID: 27618263 Free PMC article.
-
Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing.Nature. 2015 Jun 11;522(7555):231-5. doi: 10.1038/nature14408. Epub 2015 Jun 3. Nature. 2015. PMID: 26040712
-
Closing a gap in the nuclear envelope.Curr Opin Cell Biol. 2016 Jun;40:90-97. doi: 10.1016/j.ceb.2016.03.001. Epub 2016 Mar 24. Curr Opin Cell Biol. 2016. PMID: 27016712 Review.
-
The ESCRT-machinery: closing holes and expanding roles.Curr Opin Cell Biol. 2019 Aug;59:121-132. doi: 10.1016/j.ceb.2019.04.005. Epub 2019 May 24. Curr Opin Cell Biol. 2019. PMID: 31132588 Review.
Cited by
-
The interaction of Escherichia coli O157 :H7 and Salmonella Typhimurium flagella with host cell membranes and cytoskeletal components.Microbiology (Reading). 2020 Oct;166(10):947-965. doi: 10.1099/mic.0.000959. Microbiology (Reading). 2020. PMID: 32886602 Free PMC article.
-
A charged multivesicular body protein (CHMP4B) is required for lens growth and differentiation.Differentiation. 2019 Sep-Oct;109:16-27. doi: 10.1016/j.diff.2019.07.003. Epub 2019 Jul 31. Differentiation. 2019. PMID: 31404815 Free PMC article.
-
ESCRT-III mediates budding across the inner nuclear membrane and regulates its integrity.Nat Commun. 2018 Aug 23;9(1):3379. doi: 10.1038/s41467-018-05889-9. Nat Commun. 2018. PMID: 30139939 Free PMC article.
-
High-Contrast Imaging of Nanodiamonds in Cells by Energy Filtered and Correlative Light-Electron Microscopy: Toward a Quantitative Nanoparticle-Cell Analysis.Nano Lett. 2019 Mar 13;19(3):2178-2185. doi: 10.1021/acs.nanolett.9b00752. Epub 2019 Mar 4. Nano Lett. 2019. PMID: 30810045 Free PMC article.
-
CHMPions of repair: Emerging perspectives on sensing and repairing the nuclear envelope barrier.Curr Opin Cell Biol. 2020 Jun;64:25-33. doi: 10.1016/j.ceb.2020.01.011. Epub 2020 Feb 24. Curr Opin Cell Biol. 2020. PMID: 32105978 Free PMC article. Review.
References
-
- Burke B. The nuclear envelope: filling in gaps. Nat. Cell Biol. 2001;3:E273–4. - PubMed
-
- Anderson DJ, Hetzer MW. Shaping the endoplasmic reticulum into the nuclear envelope. J. Cell. Sci. 2008;121:137–142. - PubMed
-
- Burke B, Ellenberg J. Remodelling the walls of the nucleus. Nat. Rev. Mol. Cell Biol. 2002;3:487–497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

