Lipopolysaccharide Primes the NALP3 Inflammasome by Inhibiting Its Ubiquitination and Degradation Mediated by the SCFFBXL2 E3 Ligase
- PMID: 26037928
- PMCID: PMC4505057
- DOI: 10.1074/jbc.M115.645549
Lipopolysaccharide Primes the NALP3 Inflammasome by Inhibiting Its Ubiquitination and Degradation Mediated by the SCFFBXL2 E3 Ligase
Abstract
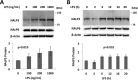
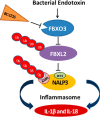
The inflammasome is a multiprotein complex that augments the proinflammatory response by increasing the generation and cellular release of key cytokines. Specifically, the NALP3 inflammasome requires two-step signaling, priming and activation, to be functional to release the proinflammatory cytokines IL-1β and IL-18. The priming process, through unknown mechanisms, increases the protein levels of NALP3 and pro-IL-1β in cells. Here we show that LPS increases the NALP3 protein lifespan without significantly altering steady-state mRNA in human cells. LPS exposure reduces the ubiquitin-mediated proteasomal processing of NALP3 by inducing levels of an E3 ligase component, FBXO3, which targets FBXL2. The latter is an endogenous mediator of NALP3 degradation. FBXL2 recognizes Trp-73 within NALP3 for interaction and targets Lys-689 within NALP3 for ubiquitin ligation and degradation. A unique small molecule inhibitor of FBXO3 restores FBXL2 levels, resulting in decreased NALP3 protein levels in cells and, thereby, reducing the release of IL-1β and IL-18 in human inflammatory cells after NALP3 activation. Our findings uncover NALP3 as a molecular target for FBXL2 and suggest that therapeutic targeting of the inflammasome may serve as a platform for preclinical intervention.
Keywords: IL-1; NALP3; cytokine induction; inflammasome; inflammation; innate immunity.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






Similar articles
-
Atrial natriuretic peptide down-regulates LPS/ATP-mediated IL-1β release by inhibiting NF-kB, NLRP3 inflammasome and caspase-1 activation in THP-1 cells.Immunol Res. 2016 Feb;64(1):303-12. doi: 10.1007/s12026-015-8751-0. Immunol Res. 2016. PMID: 26616294
-
BC-1215 inhibits ATP-induced IL-1β secretion via the FBXL2-mediated ubiquitination and degradation of not only NLRP3, but also pro-IL-1β in LPS-primed THP-1 cells.Biochem Biophys Res Commun. 2023 May 21;657:128-135. doi: 10.1016/j.bbrc.2023.03.055. Epub 2023 Mar 22. Biochem Biophys Res Commun. 2023. PMID: 37004285
-
Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver.World J Gastroenterol. 2011 Nov 21;17(43):4772-8. doi: 10.3748/wjg.v17.i43.4772. World J Gastroenterol. 2011. PMID: 22147977 Free PMC article.
-
Activation and regulation of the inflammasomes.Nat Rev Immunol. 2013 Jun;13(6):397-411. doi: 10.1038/nri3452. Nat Rev Immunol. 2013. PMID: 23702978 Free PMC article. Review.
-
The inflammasome: a danger sensing complex triggering innate immunity.Curr Opin Immunol. 2007 Dec;19(6):615-22. doi: 10.1016/j.coi.2007.09.002. Epub 2007 Oct 30. Curr Opin Immunol. 2007. PMID: 17977705 Review.
Cited by
-
Updated insights into the NLRP3 inflammasome in postoperative cognitive dysfunction: emerging mechanisms and treatments.Front Aging Neurosci. 2024 Sep 30;16:1480502. doi: 10.3389/fnagi.2024.1480502. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39411285 Free PMC article. Review.
-
Crosstalk Between the NLRP3 Inflammasome/ASC Speck and Amyloid Protein Aggregates Drives Disease Progression in Alzheimer's and Parkinson's Disease.Front Mol Neurosci. 2022 Feb 3;15:805169. doi: 10.3389/fnmol.2022.805169. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35185469 Free PMC article. Review.
-
Targeting NLRP3 Inflammasome With Nrf2 Inducers in Central Nervous System Disorders.Front Immunol. 2022 Mar 28;13:865772. doi: 10.3389/fimmu.2022.865772. eCollection 2022. Front Immunol. 2022. PMID: 35418995 Free PMC article. Review.
-
The NLRP3 inflammasome: molecular activation and regulation to therapeutics.Nat Rev Immunol. 2019 Aug;19(8):477-489. doi: 10.1038/s41577-019-0165-0. Nat Rev Immunol. 2019. PMID: 31036962 Free PMC article. Review.
-
The Role of Melatonin on NLRP3 Inflammasome Activation in Diseases.Antioxidants (Basel). 2021 Jun 24;10(7):1020. doi: 10.3390/antiox10071020. Antioxidants (Basel). 2021. PMID: 34202842 Free PMC article. Review.
References
-
- Makabe H., Kojika M., Takahashi G., Matsumoto N., Shibata S., Suzuki Y., Inoue Y., Endo S. (2012) Interleukin-18 levels reflect the long-term prognosis of acute lung injury and acute respiratory distress syndrome. J. Anesth. 26, 658–663 - PubMed
-
- Dolinay T., Kim Y. S., Howrylak J., Hunninghake G. M., An C. H., Fredenburgh L., Massaro A. F., Rogers A., Gazourian L., Nakahira K., Haspel J. A., Landazury R., Eppanapally S., Christie J. D., Meyer N. J., Ware L. B., Christiani D. C., Ryter S. W., Baron R. M., Choi A. M. (2012) Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am. J. Respir. Crit. Care Med. 185, 1225–1234 - PMC - PubMed
-
- Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B. G., Fitzgerald K. A., Hornung V., Latz E. (2009) Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 - PMC - PubMed
-
- Hershko A., Ciechanover A. (1998) The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- I01 BX002200/BX/BLRD VA/United States
- R01 HL097376/HL/NHLBI NIH HHS/United States
- R01 HL116472/HL/NHLBI NIH HHS/United States
- HL116472/HL/NHLBI NIH HHS/United States
- UH2 HL123502/HL/NHLBI NIH HHS/United States
- R01 HL125435/HL/NHLBI NIH HHS/United States
- 1UH2HL123502/HL/NHLBI NIH HHS/United States
- P01 HL114453/HL/NHLBI NIH HHS/United States
- HL098174/HL/NHLBI NIH HHS/United States
- R01 HL081784/HL/NHLBI NIH HHS/United States
- R01 HL098174/HL/NHLBI NIH HHS/United States
- HL097376/HL/NHLBI NIH HHS/United States
- HL096376/HL/NHLBI NIH HHS/United States
- HL081784/HL/NHLBI NIH HHS/United States
- P01HL114453/HL/NHLBI NIH HHS/United States
- R01 HL096376/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

