DCLAK11, a multi-tyrosine kinase inhibitor, exhibits potent antitumor and antiangiogenic activity in vitro
- PMID: 26027659
- PMCID: PMC4814203
- DOI: 10.1038/aps.2015.25
DCLAK11, a multi-tyrosine kinase inhibitor, exhibits potent antitumor and antiangiogenic activity in vitro
Abstract
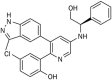
Aim: To investigate the molecular targets of DCLAK11, a novel compound discovered from a series of substituted pyridin-3-amine derivatives, and to characterize its anti-tumor properties in vitro.
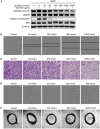
Methods: Kinase inhibition was measured by an ELISA assay. Cell viability was assessed with an SRB or a CCK8 assay. The alterations induced by kinase signaling proteins in cancer cells were detected by Western blot. Apoptosis was determined by an Annexin V-PI assay. The following assays were used to evaluate the impact on angiogenesis: wound-healing, Transwell, tube formation and microvessel outgrowth from rat aortic rings.
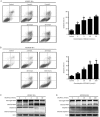
Results: DCLAK11 was a multi-targeted kinase inhibitor that primarily inhibited the EGFR, HER2, and VEGFR2 tyrosine kinases with IC50 value of 6.5, 18, and 31 nmol/L, respectively. DCLAK11 potently inhibited the proliferation of EGFR- and HER2-driven cancer cells: its IC50 value was 12 and 22 nmol/L, respectively, in HCC827 and HCC4006 cells with EGFR exon deletions, and 19 and 81 nmol/L, respectively, in NCI-N87 and BT474 cells with HER2 amplification. Consistently, DCLAK11 blocked the EGFR and HER2 signaling in cancer cells with either an EGFR or a HER2 aberration. Furthermore, DCLAK11 effectively induced EGFR/HER2-driven cell apoptosis. Moreover, DCLAK11 exhibited anti-angiogenic activity, as shown by its inhibitory effect on the proliferation, migration and tube formation of human umbilical vascular endothelial cells and the microvessel outgrowth of rat aortic rings.
Conclusions: DCLAK11 is a multi-targeted kinase inhibitor with remarkable potency against tyrosine kinases EGFR, HER2 and VEGFR2, which confirms its potent anti-cancer activity in EGFR- and HER2-addicted cancers and its anti-angiogenic activity.
Figures



Similar articles
-
SKLB610: a novel potential inhibitor of vascular endothelial growth factor receptor tyrosine kinases inhibits angiogenesis and tumor growth in vivo.Cell Physiol Biochem. 2011;27(5):565-74. doi: 10.1159/000329978. Epub 2011 Jun 15. Cell Physiol Biochem. 2011. PMID: 21691074
-
Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor.Cancer Sci. 2018 Apr;109(4):1207-1219. doi: 10.1111/cas.13536. Epub 2018 Mar 25. Cancer Sci. 2018. PMID: 29446853 Free PMC article.
-
Antiangiogenic mechanisms of PJ-8, a novel inhibitor of vascular endothelial growth factor receptor signaling.Carcinogenesis. 2012 May;33(5):1022-30. doi: 10.1093/carcin/bgs127. Epub 2012 Mar 20. Carcinogenesis. 2012. PMID: 22436611
-
Anti-angiogenic properties of artemisinin derivatives (Review).Int J Mol Med. 2017 Oct;40(4):972-978. doi: 10.3892/ijmm.2017.3085. Epub 2017 Jul 31. Int J Mol Med. 2017. PMID: 28765885 Review.
-
EGFR, HER2 and VEGF pathways: validated targets for cancer treatment.Drugs. 2007;67(14):2045-75. doi: 10.2165/00003495-200767140-00006. Drugs. 2007. PMID: 17883287 Review.
Cited by
-
Neuroprotective Efficacy of an Aminopropyl Carbazole Derivative P7C3-A20 in Ischemic Stroke.CNS Neurosci Ther. 2016 Sep;22(9):782-8. doi: 10.1111/cns.12576. Epub 2016 Jun 23. CNS Neurosci Ther. 2016. PMID: 27333812 Free PMC article.
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

