Oxidative and nitrative stress in neurodegeneration
- PMID: 26024962
- PMCID: PMC4662638
- DOI: 10.1016/j.nbd.2015.04.020
Oxidative and nitrative stress in neurodegeneration
Abstract
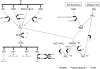
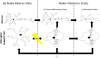
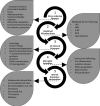
Aerobes require oxygen for metabolism and normal free radical formation. As a result, maintaining the redox homeostasis is essential for brain cell survival due to their high metabolic energy requirement to sustain electrochemical gradients, neurotransmitter release, and membrane lipid stability. Further, brain antioxidant levels are limited compared to other organs and less able to compensate for reactive oxygen and nitrogen species (ROS/RNS) generation which contribute oxidative/nitrative stress (OS/NS). Antioxidant treatments such as vitamin E, minocycline, and resveratrol mediate neuroprotection by prolonging the incidence of or reversing OS and NS conditions. Redox imbalance occurs when the antioxidant capacity is overwhelmed, consequently leading to activation of alternate pathways that remain quiescent under normal conditions. If OS/NS fails to lead to adaptation, tissue damage and injury ensue, resulting in cell death and/or disease. The progression of OS/NS-mediated neurodegeneration along with contributions from microglial activation, dopamine metabolism, and diabetes comprise a detailed interconnected pathway. This review proposes a significant role for OS/NS and more specifically, lipid peroxidation (LPO) and other lipid modifications, by triggering microglial activation to elicit a neuroinflammatory state potentiated by diabetes or abnormal dopamine metabolism. Subsequently, sustained stress in the neuroinflammatory state overwhelms cellular defenses and prompts neurotoxicity resulting in the onset or amplification of brain damage.
Keywords: Brain; Dopamine metabolism; Lipid peroxidation; Lipid/protein modification; Microglial activation; Neurodegeneration; Nitrative stress; Oxidative stress; Reactive nitrogen species; Reactive oxygen species.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia.J Neurol Sci. 2005 Jun 15;233(1-2):145-62. doi: 10.1016/j.jns.2005.03.012. J Neurol Sci. 2005. PMID: 15896810 Review.
-
Submolecular adventures of brain tyrosine: what are we searching for now?Amino Acids. 2002;23(1-3):95-101. doi: 10.1007/s00726-001-0114-6. Amino Acids. 2002. PMID: 12373523 Review.
-
Redox- and non-redox-metal-induced formation of free radicals and their role in human disease.Arch Toxicol. 2016 Jan;90(1):1-37. doi: 10.1007/s00204-015-1579-5. Epub 2015 Sep 7. Arch Toxicol. 2016. PMID: 26343967 Review.
-
Detoxification of Carbonyl Compounds by Carbonyl Reductase in Neurodegeneration.Adv Neurobiol. 2016;12:355-65. doi: 10.1007/978-3-319-28383-8_19. Adv Neurobiol. 2016. PMID: 27651263
-
A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness.Prog Neuropsychopharmacol Biol Psychiatry. 2011 Apr 29;35(3):676-92. doi: 10.1016/j.pnpbp.2010.05.004. Epub 2010 May 12. Prog Neuropsychopharmacol Biol Psychiatry. 2011. PMID: 20471444 Review.
Cited by
-
Antioxidants Attenuate Acute and Chronic Itch: Peripheral and Central Mechanisms of Oxidative Stress in Pruritus.Neurosci Bull. 2017 Aug;33(4):423-435. doi: 10.1007/s12264-016-0076-z. Epub 2016 Oct 25. Neurosci Bull. 2017. PMID: 27783328 Free PMC article.
-
MTH1 and OGG1 maintain a low level of 8-oxoguanine in Alzheimer's brain, and prevent the progression of Alzheimer's pathogenesis.Sci Rep. 2021 Mar 23;11(1):5819. doi: 10.1038/s41598-021-84640-9. Sci Rep. 2021. PMID: 33758207 Free PMC article.
-
The Interconnected Mechanisms of Oxidative Stress and Neuroinflammation in Epilepsy.Antioxidants (Basel). 2022 Jan 14;11(1):157. doi: 10.3390/antiox11010157. Antioxidants (Basel). 2022. PMID: 35052661 Free PMC article. Review.
-
Insulin Resistance and Oxidative Stress in the Brain: What's New?Int J Mol Sci. 2019 Feb 18;20(4):874. doi: 10.3390/ijms20040874. Int J Mol Sci. 2019. PMID: 30781611 Free PMC article. Review.
-
Antioxidant Activity Evaluation of Dietary Flavonoid Hyperoside Using Saccharomyces Cerevisiae as a Model.Molecules. 2019 Feb 22;24(4):788. doi: 10.3390/molecules24040788. Molecules. 2019. PMID: 30813233 Free PMC article.
References
-
- Ali TK, Matragoon S, Pillai BA, Liou GI, El-Remessy AB. Peroxynitrite mediates retinal neurodegeneration by inhibiting nerve growth factor survival signaling in experimental and human diabetes. Diabetes. 2008;57:889–898. - PubMed
-
- Ara J, Przedborski S, Naini AB, Jackson-Lewis V, Trifiletti RR, Horwitz J, Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7659–7663. - PMC - PubMed
-
- Bajo-Graneras R, Ganfornina MD, Martin-Tejedor E, Sanchez D. Apolipoprotein D mediates autocrine protection of astrocytes and controls their reactivity level, contributing to the functional maintenance of paraquat-challenged dopaminergic systems. Glia. 2011;59:1551–1566. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

